��Ŀ����
��9�֣���1�� 2010��10��1������18ʱ59��57�룬�й�̽�¶��ڹ����ȵ��ǡ��϶���š�������������գ�ȷ��졣���϶���š�����ȼ��ΪҺ���Һ����
��֪��2H2(g)��O2(g)��2H2O(g) ��H=��483.6kJ��mol��1
H2(g)��H2(l) ��H=��0.92kJ��mol��1 ��
O2(g)��O2(l) ��H=��6.84kJ��mol��1
��д��Һ���Һ��������̬ˮ���Ȼ�ѧ����ʽ_________________________________��
��2��������̽�·ɴ��������š�ʹ�õ�����ȼ�ϵ�أ����ҺΪ����������Һ���为����ӦʽΪ_________________________________________________��
��3����Ʒ���ʵ��2HCl+2Cu= CuCl2+H2����Ӧ������װ��ͼ ��
��4��pH��ͬ�����ֵ������Һ����Na2CO3 ��NaHCO3 ��CH3COONa �� NaOH��
�����ʵ���Ũ���ɴ�С��˳���ǣ����ţ� ��
��֪��2H2(g)��O2(g)��2H2O(g) ��H=��483.6kJ��mol��1
H2(g)��H2(l) ��H=��0.92kJ��mol��1 ��
O2(g)��O2(l) ��H=��6.84kJ��mol��1
��д��Һ���Һ��������̬ˮ���Ȼ�ѧ����ʽ_________________________________��
��2��������̽�·ɴ��������š�ʹ�õ�����ȼ�ϵ�أ����ҺΪ����������Һ���为����ӦʽΪ_________________________________________________��
��3����Ʒ���ʵ��2HCl+2Cu= CuCl2+H2����Ӧ������װ��ͼ ��
��4��pH��ͬ�����ֵ������Һ����Na2CO3 ��NaHCO3 ��CH3COONa �� NaOH��
�����ʵ���Ũ���ɴ�С��˳���ǣ����ţ� ��
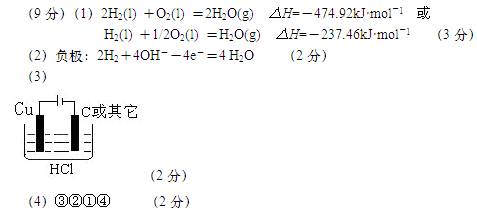
��

��ϰ��ϵ�д�
�����Ŀ
 H =-566kJ��mol��1 N2(g)+O2(g)="2NO" (g)
H =-566kJ��mol��1 N2(g)+O2(g)="2NO" (g)  CH3OH(g)��H2O(g) ��H����49.0 kJ/mol��
CH3OH(g)��H2O(g) ��H����49.0 kJ/mol��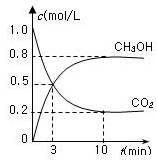
 CO2(g)+ 2H2O(l) ��H1���D 890.3 kJ/mol
CO2(g)+ 2H2O(l) ��H1���D 890.3 kJ/mol O2(g) �� H2O(g)����H1��a kJ��mol-1
O2(g) �� H2O(g)����H1��a kJ��mol-1 ����(N2H4) Ϊȼ�ϡ���������Ϊ��ȼ������֪���и����ʷ�Ӧ���Ȼ�ѧ����ʽ��
����(N2H4) Ϊȼ�ϡ���������Ϊ��ȼ������֪���и����ʷ�Ӧ���Ȼ�ѧ����ʽ��