题目内容
已知:(1)Zn(s)+1/2O2(g)=ZnO(s);△H=-348.3 kJ/mol;
(2)2Ag(s)+1/2O2(g)=Ag2O(s);△H=-31.0 kJ/mol。则Zn(s)+Ag2O(s)=ZnO(s)+2Ag(s)的△H等于
(2)2Ag(s)+1/2O2(g)=Ag2O(s);△H=-31.0 kJ/mol。则Zn(s)+Ag2O(s)=ZnO(s)+2Ag(s)的△H等于
| A.-317.3 kJ/mol | B.-379.3 kJ/mol |
| C.-332.8 kJ/mol | D.317.3 kJ/mol |
A
将方程式(1)Zn(s)+1/2O2(g)=ZnO(s);△H=-348.3 kJ/mol;(2)2Ag(s)+1/2O2(g)=Ag2O(s);△H=-31.0 kJ/mol;两方程式叠加,可得Zn(s)+Ag2O(s)=ZnO(s)+2Ag(s);△H=-317.3 kJ/mol

练习册系列答案
 口算题卡加应用题集训系列答案
口算题卡加应用题集训系列答案
相关题目
 (2)2Zn(s)+O2(g)=2ZnO(s) Δ
(2)2Zn(s)+O2(g)=2ZnO(s) Δ H1 =" —702" kJ/mol
H1 =" —702" kJ/mol 2Hg(l)+O2(g)=2HgO(s) ΔH2 =" —182" kJ/mol
2Hg(l)+O2(g)=2HgO(s) ΔH2 =" —182" kJ/mol
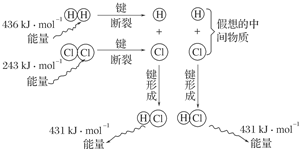
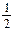 H2 (g) +
H2 (g) +