��Ŀ����
��ѧ��Ӧ����Ϊ�ɻ�ѧ�����Ѻ��»�ѧ���γɵĹ��̡���ѧ���ļ������γɣ����1 mol��ѧ��ʱ�ͷţ������գ�����������֪��N��N���ļ�����a kJ?mol��1��H��H���ļ�����b kJ?mol��1����N2��H2�ϳ�1 mol NH3ʱ�ɷų�c kJ����������ôN��H ���ļ�����(����)
| A����a��3b��2c��/2 kJ?mol��1 | B����a��3b��2c��/6 kJ?mol��1 |
| C����a��3b��2c��/6 kJ?mol��1 | D����a��3b��2c��/3 kJ?mol��1 |
B
�ϳɰ��ķ�Ӧ����ʽΪ��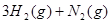 ?
?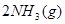 ��H =
��H =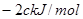 ����N��H ���ļ���Ϊ
����N��H ���ļ���Ϊ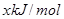 ������
������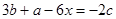 �����
�����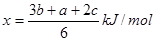 ��
��
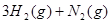 ?
?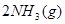 ��H =
��H =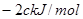 ����N��H ���ļ���Ϊ
����N��H ���ļ���Ϊ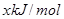 ������
������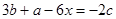 �����
�����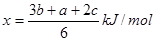 ��
��
��ϰ��ϵ�д�
�����Ŀ
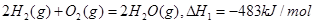 ��������Ȼ�ѧ����ʽ��
��������Ȼ�ѧ����ʽ��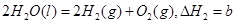 ������˵����ȷ���ǣ� ��
������˵����ȷ���ǣ� ��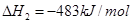
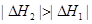
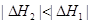
 ˮʱ����22.68kJ�����ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽΪ________________ ________________��
ˮʱ����22.68kJ�����ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽΪ________________ ________________��
 2HBr(g) ��H=��72kJ/mol������1mol Br2(1)��Ҫ���յ�����Ϊ30kJ����������������±���
2HBr(g) ��H=��72kJ/mol������1mol Br2(1)��Ҫ���յ�����Ϊ30kJ����������������±��� l����ȫȼ�������ȶ�������ų�������ΪY kJ����1molP��O2��Ӧ���ɹ�̬P2O3�ķ�Ӧ�ȡ�H=________________________________��
l����ȫȼ�������ȶ�������ų�������ΪY kJ����1molP��O2��Ӧ���ɹ�̬P2O3�ķ�Ӧ�ȡ�H=________________________________��