��Ŀ����
���칬һ�š����й���һ��Ŀ�����������2011��9��29����������ʽ���������������Բ������ɹ������չʾ���ҹ�����ˮƽ���ۺ�ʵ��������ƽ������� ����(N2H4) Ϊȼ�ϡ���������Ϊ��ȼ������֪���и����ʷ�Ӧ���Ȼ�ѧ����ʽ��
����(N2H4) Ϊȼ�ϡ���������Ϊ��ȼ������֪���и����ʷ�Ӧ���Ȼ�ѧ����ʽ��
N2H4(g)+O2(g)=N2(g)+2H2O(g)����H1=��533.23 kJ��mol��1
H2O(g)��H2O (l) �� ��H2���C44 kJ��mol��1
2H2O2(l)��2H2O(l)+O2(g) ��H3���C196.4 kJ��mol��1
��������������ⷴӦ���Ȼ�ѧ����ʽ�ɱ�ʾΪ
 ����(N2H4) Ϊȼ�ϡ���������Ϊ��ȼ������֪���и����ʷ�Ӧ���Ȼ�ѧ����ʽ��
����(N2H4) Ϊȼ�ϡ���������Ϊ��ȼ������֪���и����ʷ�Ӧ���Ȼ�ѧ����ʽ��N2H4(g)+O2(g)=N2(g)+2H2O(g)����H1=��533.23 kJ��mol��1
H2O(g)��H2O (l) �� ��H2���C44 kJ��mol��1
2H2O2(l)��2H2O(l)+O2(g) ��H3���C196.4 kJ��mol��1
��������������ⷴӦ���Ȼ�ѧ����ʽ�ɱ�ʾΪ
| A��N2H4(g)+2H2O2(l)= N2(g)+4H2O(l)��H����817.63 kJ��mol��1 |
| B��N2H4(g)+2H2O2(l)= N2(g)+4H2O(g)��H����817.63 kJ��mol��1 |
| C��N2H4(g)+2H2O2(l)= N2(g)+4H2O(l)��H����641.63 kJ��mol��1 |
D��N2H4(g)+2H2O2(l)= N2(g)+4H2O( g)��H����641.63 kJ��mol��1 g)��H����641.63 kJ��mol��1 |
AD
��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 ˮʱ����22.68kJ�����ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽΪ________________ ________________��
ˮʱ����22.68kJ�����ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽΪ________________ ________________�� H =" " ��142.9kJ��mol��l
H =" " ��142.9kJ��mol��l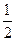 O 2(g) = H2O(1)
O 2(g) = H2O(1) 4NO(g)��6H2O(g)�� ��H����905 kJ/mol������
4NO(g)��6H2O(g)�� ��H����905 kJ/mol������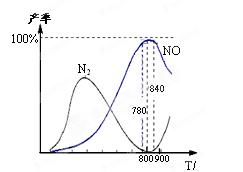
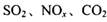 �ǶԻ���Ӱ��ϴ�Ķ������壬�����ǵĺ������ƺ��������Ż��������滷������Ч;����
�ǶԻ���Ӱ��ϴ�Ķ������壬�����ǵĺ������ƺ��������Ż��������滷������Ч;���� Ũ�ȵ���__________ (ѡ����ĸ)��
Ũ�ȵ���__________ (ѡ����ĸ)��


 �����£�
�����£�
 ,��ƽ�ⳣ��K=13.3��
,��ƽ�ⳣ��K=13.3�� ����
���� =_______________(������λ��Ч����)��
=_______________(������λ��Ч����)��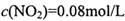 ��
�� ����ı��������____________________
����ı��������____________________