��Ŀ����
����Ŀ��A��B��C��D��E��Ԫ�����ڱ���ԭ���������������ǰ������Ԫ�ء�A��B��Cͬ���ڣ���AԪ��ԭ�Ӻ���s�ܼ���1��δ�ɶԵ��ӣ�CԪ�ص�ԭ��������B��1����CԪ�ص�ԭ�Ӻ���p���ӱ�s������1��DԪ�ص�ԭ�Ӽ۵�����6��δ�ɶԵ��ӣ�����һ�ֻ����ﳣ���ڼ���Ƽݣ�EԪ�ص�ԭ������Ϊ27����ش��������⣺
��1����һ�����ܽ���A��C֮���ͬ����Ԫ����________________����Ԫ�ط��ţ���
��2��CԪ�ص��������������Ӧ��ˮ������ˮ��Һ������ȫ���룬�������������ӵĿռ乹��Ϊ____________��д������һ����������ӻ�Ϊ�ȵ���������Ļ�ѧʽ___________��
��3����BԪ��ͬ�����Ԫ�ع裬����±����ķе��Ǧ�Ķ�±�����۵���ͼ��ʾ��
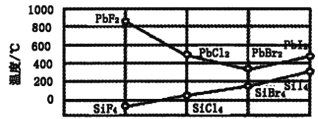
��SiX4�ķе���F��Cl��Br��I�������ߵ�ԭ����_________________________��
�ڽ��PbX2���۵�ı仯���ɣ��ƶ��������ʵ��۵�ߵͣ�NaCl_______MgO������<������>������=������
��4��Ԫ��D�����γ���ͼ��ʾ���������������������ѧ�������Ͳ�����________������ĸ����
a�����Թ��ۼ� b���Ǽ��Թ��ۼ� c����λ�� d�����Ӽ�
e�������� f������ g������
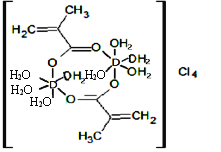
��5����֪E3+���γ���λ��Ϊ6���������гȻ�ɫ���Ϻ�ɫ����E����������ɾ��ɱ�ʾΪECl3��nNH3��Ϊ�ⶨ����������ᄃ��Ļ�ѧʽ�����������ʵ��:
a���ֱ�ȡ�����������־������Ʒ�����Һ��
b����������Һ�зֱ����AgNO3��Һ����������ɫ������
c��������ȫ��ֱ���ˣ���ϴ�Ӹ��������������Ϻ�ɫ�������������������Ȼ�ɫ�����������������֮��Ϊ2��3 �����Ϻ�ɫ����Ļ�ѧʽΪ______________������������ʽд����ѧʽ����E��Ԫ�ط��ű�ʾ��n�þ������ֱ�ʾ����
���𰸡�Be��B��C��O ƽ�������� CO32- ��SO3 ��ɺͽṹ���Ƶķ��ӣ�������Է������������»�����ǿ���е����� < e [Co(NH3)5Cl]Cl2
��������
A��B��C��D��E��Ԫ�����ڱ���ԭ���������������ǰ������Ԫ�ء�DԪ�ص�ԭ�Ӽ۵�����6��δ�ɶԵ��ӣ�ֻ�����ڹ���Ԫ�أ��۵����Ų�ʽΪ3d54s1����DΪCr��EԪ�ص�ԭ������Ϊ27����EΪCo��CԪ�ص�ԭ�Ӻ���p���ӱ�s������1����������Ų�ʽΪ1s22s22p3����CΪNԪ�ء�A��B��Cͬ���ڣ�CԪ�ص�ԭ��������B��1����BΪCԪ�أ�AԪ��ԭ�Ӻ���s�ܼ���1��δ�ɶԵ��ӣ���AΪLiԪ�ء�
(1)ͬ����Ԫ�أ���ԭ�����������һ�����ܳ��������ƣ���IIA�塢VA��ĵ�һ�����ܸ���ͬ��������Ԫ�أ�
(2)CԪ�ص�����������ˮ������ˮ��Һ������ȫ���룬������ΪNO3-����ϼ۲���Ӷ���=�������Ӷ���+![]() (a-xb)�����жϣ���ϵȵ�����ĸ�������жϣ�
(a-xb)�����жϣ���ϵȵ�����ĸ�������жϣ�
(3)��SiX4��ɽṹ���ƣ���Ϊ���Ӿ��壬������Է������������Ӽ������������۷е����ߣ�����ͼ��֪��PbX2�Ļ�ѧʽ���ƣ���PbF2��PbCl2���۵��PbBr2��PbI2�ĸߣ�˵�����ǵľ������Ͳ�ͬ����ϸ������͵ľ�����۵�ߵ͵��жϷ����жϣ�
(4)̼ԭ��֮���γɷǼ��Լ���C��Hԭ�ӡ�Oԭ��֮���γɼ��Լ���ˮ������O��Hԭ��֮��Ҳ�γɼ��Լ���Oԭ����Cr֮���γ���λ�����ڽ������������������֮���γ����Ӽ�������Ϊ������˫����������������
(5)Co3+����λ����Ϊ6����������Һ�е���AgNO3��Һ����������ɫ��������������������������硣���ݳ�������ϴ�Ӹ����������Ϻ�ɫ�������������������Ȼ�ɫ�����������������֮��Ϊ2��3����֪�Ϻ�ɫ��������2������������磬�Ȼ�ɫ��������3������������磬�ݴ��ƶϻ�ѧʽ��
��������������AΪLiԪ�أ�BΪCԪ�أ�CΪNԪ�أ�DΪCrԪ�أ�EΪCoԪ�ء�
(1)ͬ����Ԫ�أ���ԭ�����������һ�����ܳ��������ƣ���IIA�塢VA��ĵ�һ�����ܸ���ͬ��������Ԫ�أ��ʵ�һ�����ܣ�N��O��C��Be��B��Li����һ�����ܽ���A��C֮���ͬ����Ԫ����Be��B��C��O���ʴ�Ϊ��Be��B��C��O��
(2)CԪ�ص�����������ˮ������ˮ��Һ������ȫ���룬������ΪNO3-��NO3-��Nԭ�Ӽ۲���Ӷ���=3+![]() =3���µ��Ӷԣ�VSEPRģ�ͼ�Ϊ���ӿռ乹�ͣ���NO3-�Ŀռ乹��Ϊ��ƽ���������Σ���������ӻ�Ϊ�ȵ����������������Sԭ���滻Nԭ����1����λ����ɣ�������Cԭ�ӡ�1����λ������滻Nԭ�ӣ����ֵȵ�������ΪSO3��CO32-�ȣ��ʴ�Ϊ��ƽ���������Σ�SO3(��CO32-)��
=3���µ��Ӷԣ�VSEPRģ�ͼ�Ϊ���ӿռ乹�ͣ���NO3-�Ŀռ乹��Ϊ��ƽ���������Σ���������ӻ�Ϊ�ȵ����������������Sԭ���滻Nԭ����1����λ����ɣ�������Cԭ�ӡ�1����λ������滻Nԭ�ӣ����ֵȵ�������ΪSO3��CO32-�ȣ��ʴ�Ϊ��ƽ���������Σ�SO3(��CO32-)��
(3)��SiX4�۷е������ߣ�SiX4��ɺͽṹ���ƣ���Ϊ���Ӿ��壬������Է������������Ӽ������������۷е����ߣ��ʴ�Ϊ��SiX4��Ϊ��ɺͽṹ���Ƶķ��ӣ�������Է������������Ӽ���������ǿ���е����ߣ�
����ͼ��֪��PbX2�Ļ�ѧʽ���ƣ���PbF2��PbCl2���۵��PbBr2�ĸߣ�˵��PbF2��PbCl2Ϊ���Ӿ��壬PbBr2��PbI2���۵�ݱ������SiX4���۵�ݱ�������ƣ���PbBr2��PbI2Ϊ���Ӿ��塣���Ӿ��������ӵĵ��Խ�࣬���Ӱ뾶ԽС��������Խ���۵�Խ�ߣ�����۵㣺NaCl��MgO���ʴ�Ϊ������
(4)̼ԭ��֮���γɷǼ��Լ���C��Hԭ�ӡ�Oԭ��֮���γɼ��Լ���ˮ������O��Hԭ��֮��Ҳ�γɼ��Լ���Oԭ����Cr֮���γ���λ�����ڽ������������������֮���γ����Ӽ�������Ϊ������˫����������������û�н��������ʴ�Ϊ��e��
(5)Co3+����λ����Ϊ6����������Һ�е���AgNO3��Һ����������ɫ��������������������������硣���ݳ�������ϴ�Ӹ����������Ϻ�ɫ�������������������Ȼ�ɫ�����������������֮��Ϊ2��3����֪�Ϻ�ɫ��������纬2�������ӣ��Ȼ�ɫ��������纬3�������ӣ����Ϻ�ɫ����Ļ�ѧʽΪ[Co(NH3)5Cl]Cl2���ʴ�Ϊ��[Co(NH3)5Cl]Cl2��

 ����ʦ��Сһ����ʦ������ҵϵ�д�
����ʦ��Сһ����ʦ������ҵϵ�д�����Ŀ���û���̿��ԭ�����Դ�����������練Ӧ��![]()
![]() ����
����![]() ʱ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
ʱ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
0 | 10 | 20 | 30 | 40 | 50 | |
NO | 1.00 | 0.58 | 0.40 | 0.40 | 0.48 | 0.48 |
N2 | 0 | 0.21 | 0.30 | 0.30 | 0.36 | 0.36 |
CO2 | 0 | 0.21 | 0.30 | 0.30 | 0.36 | 0.36 |
(1)T1��ʱ���÷�Ӧ��ƽ�ⳣ��![]() ______
______
(2)30min��ֻ�ı�ijһ��������Ӧ���´ﵽƽ�⣬�����ϱ��е������жϸı������������ ______ ![]() ��һ�ּ���
��һ�ּ���![]()