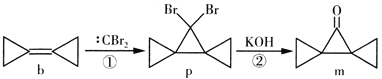
��Ŀ����
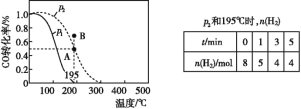
����Ŀ���������Һ�絼��Խ������Խǿ����������0.100 mol��L��1����ֱ�ζ�10.00 mLŨ�Ⱦ�Ϊ0.100 mol��L��1��NaOH��Һ�Ͷ��װ�[(CH3)2NH]��Һ(���װ���ˮ�е����백���ƣ�����Kb[(CH3)2NH��H2O]��1.6��10��4]�����ô�������õζ���������Һ�ĵ絼����ͼ��ʾ������˵������ȷ����(����)
A.���ߢڴ����ζ����װ���Һ������
B.b����Һ�У�pH<7
C.d����Һ�У�c(H��)��c(OH��)��c[(CH3)2NH��H2O]
D.c��d��e�������Һ�У�ˮ�ĵ���̶�������d��
���𰸡�B
��������
�������Һ�絼��Խ������Խǿ���絼��������Ũ�ȳ����ȣ�����Ũ��Խ��絼��Խ������Һ������Խǿ����δ��HCl��Һʱ���ٵĵ絼�ʴ��ڢڣ�˵������ǿ�����������NaOH��Һ�����Ƕ��װ���Һ��
A���ɷ�����֪�����ߢ�Ϊ���װ���Һ�ı仯���ߣ���A��ȷ��
B��b����Һ�е�����Ϊ(CH3)2NH��H2O�ͣ�CH3��2NH2Cl�������ʵ���֮��Ϊ1:1��Kb[(CH3)2NH��H2O]��1.6��10��4����Kh[(CH3)2NH2+] =KW/Kb=10-14/1.6��10��4=6.25��10��9������Һ�е���̶ȴ���ˮ��̶ȣ���Һ�ʼ��ԣ�����pH>7,��B����
C. d����Һ������Ϊ��CH3��2NH2Cl�����������غ��֪��c(H��)��c(OH��)��c[(CH3)2NH��H2O]����C��ȷ��
D.��������ˮ���룬���������ӵ��δٽ�ˮ���룬c����Һ������ΪNaCl��d����Һ������Ϊ��CH3��2NH2Cl��e����Һ������Ϊ��CH3��2NH2Cl��HCl���Ҷ���Ũ����ȣ�c��ˮ�ĵ���û��Ӱ�졢 e����ˮ���롢d�ٽ�ˮ���룬����ˮ����̶�������d�㣬��D��ȷ��
�ʴ�ΪB��

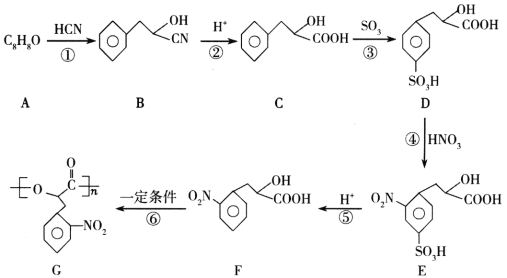
����Ŀ��һ���¶��£���3�������Ϊ1L�ĺ����ܱ������з�����ӦCO(g)+H2S(g)COS(g)+H2(g)��H=akJmol-1�ﵽƽ�⡣����˵������ȷ����
������� | �¶�/K | ���ʵ���ʼŨ��/molL-1 | ���ʵ�ƽ��Ũ��/molL-1 | |||
CO(g) | H2S(g) | COS(g) | H2(g) | COS(g) | ||
1 | T1 | 10.0 | 7.0 | 0 | 0 | 2.0 |
2 | T1 | 5.0 | 3.5 | 0 | 0 | |
3 | T2 | 3.0 | 0 | 7.0 | 7.0 | 1.5 |
A.��T1��T2����a��0
B.T1Kʱ���÷�Ӧ��ƽ�ⳣ��K=0.1
C.����3�з�Ӧ�ﵽƽ����ٳ���1.1molH2S(g)��0.3molH2(g)��ƽ�ⲻ�ƶ�
D.����1��H2S��ƽ��ת���ʱ�����2С
����Ŀ��ijѧ������֪���ʵ���Ũ�ȵ��������ζ�δ֪���ʵ���Ũ�ȵ�����������Һʱ��ѡ���̪��ָʾ��������д���пհף�
��1�����ƴ���Һ����5.0g�����������ʣ����ʲ������ᷴӦ���Ĺ����ռ���Ʒ����1000mL��Һ�����ձ�����Ͳ�ͽ�ͷ�ι��⣬����Ҫ�IJ���������__________��__________��
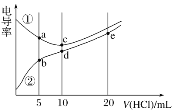
��2���ζ����ñ���������Һ�ζ����������������Һʱ���ﵽ�ζ��յ���ж������ǣ�__________�����ζ���ʼ�ͽ���ʱ����ʽ�ζ����е�Һ����ͼ��ʾ�����յ����_________mL��
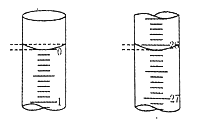
��3�����ݴ�����ijѧ����������ʵ��ֱ��¼�й��������£�
�ζ����� | ��������������Һ�����/mL | 0.1000mol/L����������mL�� | |
�ζ�ǰ�̶� | �ζ���̶� | ||
��һ�� | 25.00 | 0.00 | 26.11 |
�ڶ��� | 25.00 | 1.56 | 30.30 |
������ | 25.00 | 0.22 | 26.31 |
�������������Һ�����ʵ���Ũ��c��NaOH��=___________����������λ��Ч���֣�
��4�������ú�Na2O���ʵ�NaOH���Ƴɱ���Һ���ζ����ᣬ��õ�����Ũ�Ƚ�________����ѡ����ƫ��������ƫ����������Ӱ������