��Ŀ����
����Ŀ�������أ������ĺ��������Ϊ��ɫ��״���壬�����ڱ�ͪ���ȷºͱ��У��ڼ״����Ҵ������ѡ�ʯ�����п��ܽ⣬��ˮ�м������ܣ��۵�Ϊ156��157�棬���ȶ��Բ�������Ǹ�Ч�Ŀ�űҩ����֪�����ѷе�Ϊ35�档����������ȡ�����صķ���֮һ������ȡԭ��Ϊ�����ģ���Ҫ�����ѽ�ȡ�������ͽ�ȡ�������ѽ�ȡ������Ҫ����Ϊ��

��ش��������⣺
(1)��������и�������Ŀ����_______��
(2)����I��Ҫ�IJ���������Ҫ�У��ձ���©����______���������������_____��
(3)���������Ҫ���̿�����_______(����ĸ)��
A.��ˮ�ܽ⣬����Ũ������ȴ�ᾧ
B.��95%���Ҵ���Ũ�����ᾧ������
C.�������ѽ�����ȡ��Һ
(4)ijѧ���������ص����ʽ���̽�����������ؼ��뺬��NaOH����̪��ˮ��Һ�У������ص��ܽ�����С�����Ȳ����裬�����ص��ܽ�����������Һ��ɫ��dz��˵����������_____(����ĸ)������ͬ�����ʡ�
A.�Ҵ� B.���� C.�������� D.������
���𰸡��������������ѵĽӴ��������������صĽ�ȡ�� ������ ���� B C
��������
�������ѽ�ȡ�������̿�֪����������и�����飬�����������������ѵĽӴ��������������صĽ�ȡ�ʣ������Ѷ������ؽ��н�ȡ���ˣ��ɵ���Һ����������ȡҺ�������ɵ������صĴ�Ʒ���ٶԴ�Ʒ��95%���Ҵ�����Ũ�����ᾧ�����˿ɵþ�Ʒ��
(1)�������ѽ�ȡ�������̿�֪����������и�����飬�����������������ѵĽӴ�������Ӷ�����������صĽ�ȡ�ʣ�
(2)�������̷���������I�Ƿ��������Թ����������Һ������IJ�������������Ϊ���ˣ���Ҫʹ�õIJ���������Ҫ�У��ձ���©�������������������Ƿ��뻥�ܵķе㲻ͬ��Һ������ķ���������������
(3)��������ķ�����֪�����Ʒ�м�95%���Ҵ�����Ũ�����ᾧ�����˿ɵþ�Ʒ���ʺ���ѡ����B��
(4)������������ˮ��������������������ˮ��Һ���ܽ⣬������¶ȶ������������Ƶ����ʵ�����Ӱ���֪���������к���������ѡ���к��е�����ֻ�������������ʺ���ѡ����C��

����Ŀ����ʷ�Ͻ�����ͭ��Ҫ����Ϊ���ҽ�����װ��Ʒ��Ӧ�á��Իش��������⣺
(1)Ag��Ԫ�����ڱ��е�λ��_________��Ag���ļ۵����Ų�ʽΪ_________��
(2)ұ��ҵ�ϣ���ȡ���ԭ����2[Au(CN)2]����Zn��2Au��[Zn(CN)4]2������CN����Ϊ�ȵ�����ķ�����________(��дһ��)��HCN������������������Ŀ֮��Ϊ_________��
(3)��֪��̬ͭ�IJ��ֵ����������ʾ��
������/kJ/mol | I1 | I2 | I3 |
Cu | 746 | 1958 | 2058 |
�ɱ�������֪��I2(Cu)ԶԶ����I1(Cu)����ԭ����_________��
(4)��֪������ͭ��Һ�е��백��������(H2N��CH2��COONa)���ɵõ��ṹ��ͼ��ʾ����
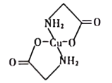
���������̼ԭ�ӵĹ���ӻ�����Ϊ_________��
��1mol����������(H2N��CH2��COONa)������������ĿΪ_________mol��
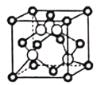
(5)ijQԭ�ӵ���Χ�����Ų�ʽΪ3s23p5��ͭ��Q�γɻ�����ľ�����ͼ��ʾ(�������Qԭ��)��ÿ��ͭԭ����Χ���������ͭԭ����ĿΪ_________�����þ���Ļ�ѧʽΪ_________����֪�þ�����ܶ�Ϊ��g��cm��3�������Ħ������ΪMg/mol������٤��������ֵΪNA����þ�����ͭԭ�Ӻ�Qԭ��֮�ʵ���̾���Ϊ_________pm(1pm��10��12m��ֻд����ʽ)��
����Ŀ�����и���ʵ��������������ó��Ľ�����ȷ���ǣ� ��
ѡ�� | ʵ����� | ʵ������ | ���� |
A | ������SO2��BaCl2��Һ��ͨ������X | ������ɫ���� | Xһ�������������� |
B | ��NaAlO2��Һ�г���ͨ������Y | �ȳ��ְ�ɫ���������ճ������ܽ� | Y������CO2���� |
C | ��Na2CO3��Һ�м�������ᣬ������������ֱ��ͨ�뱽������Һ�� | ������ɫ���� | ���ԣ�����>̼��>���� |
D | ��ʢ��KI3��Һ�����Թ��зֱ���������Һ��AgNO3��Һ | ǰ����Һ����ɫ�������л�ɫ���� | KI3��Һ�д���I3- |
A.AB.BC.CD.D
����Ŀ����ҵ�ϳ���PCl5��Ϊ����������ά�صĴ�����Ҳ����������ҩƷ���з����ڲ��ϣ�ҽҩ��ҵ�õ��˹㷺Ӧ�ã���ش��������⣺
(1)PCl5�ڹ�̬�����ӻ�������ʽ��Ϊ417����������Ϊ![]() ���������ӵĻ�ѧʽΪ_______��
���������ӵĻ�ѧʽΪ_______��
(2)��֪�� P(s) + ![]() Cl2(g) = PCl3(g) H= -306 KJ/mol
Cl2(g) = PCl3(g) H= -306 KJ/mol
PCl5(g) = PCl3(g) + Cl2(g) H= + 93 KJ/mol
��Ӧ2P(s) + 5Cl2(g) = 2PCl5(g)��H=______kJ/mol��
(3)�¶�ΪTʱ����2.0L�����ܱ������г���1.0 molPCl5�������з�����Ӧ��PCl5(g)PCl3(g)+Cl2(g)����һ��ʱ���ﵽƽ�⣮��Ӧ�����вⶨ�IJ������ݼ��±���
t/min | 0 | 10 | 20 | 30 | 40 |
n(PCl3)/mol | 0 | 0.12 | 0.17 | 0.2 | 0.2 |
�ٷ�Ӧ��ǰ10min��ƽ������v(PCl5)=_______��
�ڲ��ܱ����÷�Ӧ�ﵽƽ��״̬���ǣ�____ (ѡ�����)��
a����������ѹǿ���ٸı�
b����������ƽ����Է�����������
c��v��(PCl3)����v��(Cl2)
d�����������ܶȲ���
e��PCl5���������ٱ仯
��Ҫ���������Ӧ��ת���ʣ��ɲ�ȡ�Ĵ�ʩ��(��������)___________��
�ܸ��¶��£���ӦPCl5(g)PCl3(g) + Cl2(g)��ƽ�ⳣ��Kp=2.25��105(KpΪѹǿƽ�ⳣ����Kp=![]() )����һ������PCl5����һ���ƿ�ڣ���ƽ���PCl5�ķ�ѹΪ2.5��104Pa����PCl5�ķֽ���Ϊ________��
)����һ������PCl5����һ���ƿ�ڣ���ƽ���PCl5�ķ�ѹΪ2.5��104Pa����PCl5�ķֽ���Ϊ________��
(4)����ˮ�У����Ȼ�����ȫˮ�⣬������������ᡣ����0.01molPCl5Ͷ����ˮ�����lL��Һ������μ���AgNO3��Һ���Ȳ����ij�����_____�� [��֪Ksp(Ag3PO4)=1.4��10-16��Ksp(AgCl)=1.8��10-10]��