��Ŀ����
3����֪ij���л���A������ͼ�ͺ������ͼ��ͼ��ʾ��
�ش��������⣺
��1�����������ͼ�ƶϣ��л���A����Է�������Ϊ74������ʽΪC3H6O2��
���л���������������Һ�ڼ��������·�Ӧ�Ļ�ѧ����Ϊ��CH3COOCH3+NaOH$\stackrel{��}{��}$CH3COONa+CH3OH��
��2������B����Է���������AС4����2����������ʹ������Ȼ�̼��Һ��ɫ��д��B���п��ܵĽṹ��ʽ������˳���칹����
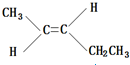 ��
��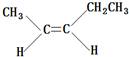 ��
��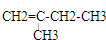 ��
��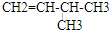 ��
����3������C��B��ͬϵ��������ϩ������ܶ�Ϊ3��C�ĺ˴Ź���������ʾ��C��ֻ����һ����ԭ�ӣ���C��һ�������·����ۺϷ�Ӧ�ķ���ʽΪ��
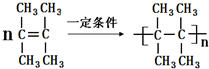 ��
��
���� ��1������ͼ���ұߵķ������ӷ��ʾ�����ʵ���Է����������ɺ������ͼ�ɿ����÷������в��Գ�CH3����˸÷�������2��CH3����ͼҲ���Կ�������C=O˫����C-O-C��������дA�Ľṹʽ���ݴ˽��
��2��A����Է�������Ϊ74������B����Է�������Ϊ70��B��ʹ������Ȼ�̼��Һ��ɫ��B�к��в����ͼ�������Է���������֪BΪϩ��������̼ԭ����Ŀ�ж�B�ķ���ʽ������2��������˫���ڶ�λ�ã�����1��֧����֧���в���֧��������˫�����ٶ�λ�ã���֧�����ݴ���д��
��3������C��B��ͬϵ��������ϩ������ܶ�Ϊ3����C�ķ���ʽΪC6H12��C�ĺ˴Ź���������ʾ��C��ֻ����һ����ԭ�ӣ���ΪC=C˫������4������
��� �⣺��1������ͼ���ұߵķ������ӷ��ʾ�����ʵ���Է�����������������ͼ��֪�����л������Է�������Ϊ74���ɺ������ͼ�ɿ����÷������в��Գ�CH3����˸÷�������2��CH3����ͼҲ���Կ�������C=O˫����C-O-C����������A�Ľṹ��ʽΪCH3COOCH3������ʽΪC3H6O2��CH3COOCH3������������Һ�ڼ��������£�����ˮ�ⷴӦ���������ơ��״�����Ӧ����ʽΪ��CH3COOCH3+NaOH$\stackrel{��}{��}$CH3COONa+CH3OH��
�ʴ�Ϊ��74��C3H6O2��CH3COOCH3+NaOH$\stackrel{��}{��}$CH3COONa+CH3OH��
��2��A����Է�������Ϊ74������B����Է�������Ϊ70��B��ʹ������Ȼ�̼��Һ��ɫ��B�к��в����ͼ�������Է���������֪BΪϩ������B�ķ���ʽΪCnH2n����14n=70������n=5����B�ķ���ʽΪC5H10��B����2��������˫���ڶ�λ�ã�����1��֧����֧���в���֧������B���ܵĽṹ��ʽΪ ��
�� ����˫�����ٶ�λ�ã���֧����B���ܵĽṹ��ʽΪ
����˫�����ٶ�λ�ã���֧����B���ܵĽṹ��ʽΪ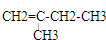 ��
��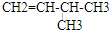 ��
��
�ʴ�Ϊ�� ��
�� ��
��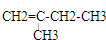 ��
��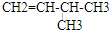 ��
��
��3������C��B��ͬϵ��������ϩ������ܶ�Ϊ3����C�ķ���ʽΪC6H12��C�ĺ˴Ź���������ʾ��C��ֻ����һ����ԭ�ӣ���ΪC=C˫������4������C�Ľṹ��ʽΪ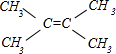 �������Ӿ۷�Ӧ�ķ���ʽΪ��
�������Ӿ۷�Ӧ�ķ���ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
���� ���⿼���л���ṹʽ��ȷ����ͬ���칹����д��֪ʶ����Ŀ�Ѷ��еȣ���������ͼȷ��A����Է��������ǹؼ���ע�����֪ʶ�����գ�����������ѧ���ķ������������������Ӧ�û���֪ʶ��������

| A�� | Ħ���ǰѿɳ�����������������ϵ������һ�����ʻ��������� | |
| B�� | �����Ħ��������¶Ⱥ�ѹǿ�йأ��¶�Խ�ߣ����Խ�� | |
| C�� | �κ�һ�������ӣ���Ħ��������g/molΪ��λ����ֵ���������ӵ���Է������������ԭ��������ͬ | |
| D�� | ��x��N������ԭ�ӵ�������1g�����ӵ������ɱ�ʾΪ14x mol-1 |
| A�� | HCl��MgCl2 | B�� | Na��Na+ | C�� | CO��CO2 | D�� | Fe3+��Fe |
| A�� | ��״���£�22.4LCH4��CH2C12�Ļ��������������ΪNA | |
| B�� | �ú�0.2mo1 FeC13�ı�����Һ�Ƶý��壬������������������������ĿΪ0.2 NA | |
| C�� | ��NO2��CO2��ɵĻ�������й���NA�����ӣ�������Ԫ�ص�����һ��Ϊ32g | |
| D�� | 2LpH=1��HA����Һ������п����Ӧ������H2�ķ�����һ��Ϊ0.1 NA |
| ���� | Al | Al2O3 | Fe | Fe2O3 |
| �۵�/�� | 660 | 2054 | 1535 | 1462 |
| �е�/�� | 2467 | 2980 | 2750 |
��1��д�����ȷ�Ӧ�Ļ�ѧ����ʽ��2Al+Fe2O3$\frac{\underline{\;����\;}}{\;}$2Fe+Al2O3��
��2���ӱ��������Ʋ����ȷ�Ӧ���õ����������������Ͻ�����ɣ�Al���۵��Fe�ĵͣ�
��3����������֪ʶд��һ����֤��������Fe����������ô��������������������˵���������ɣ�
��4�����һ����ʵ�鷽����֤���������õ��������к��н���������ֻ�÷�Ӧ���ӷ���ʽ��ʾ����2Al+2OH-+2H2O=2AlO2-+3H2����
��5�������ȷ�Ӧ�У��������õù��࣬�ڷ�Ӧ�ɻ��յ������������Ļ���������15g�û�������150mLϡ�����У��ڱ�״���·ų�1.68L������Ϊ�к��������ᣬ��ʹ��Һ�е�Al3+ǡ����ȫת��ΪAl��OH��3��������Ҫ200mLŨ��Ϊ6mol•L-1��NaOH��Һ��ͬ��ϡ��������ʵ���Ũ����4mol/L��
| A�� | Naԭ���������1������ | B�� | Na�����ܸ�ˮ��Ӧ�ų����� | ||
| C�� | Naԭ�ӱ�Mgԭ�Ӹ���ʧȥ���� | D�� | Na�������ڼ������������������� |
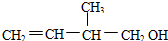 ���е�����������ǣ�������
���е�����������ǣ�������| A�� | ���л���Ĺ��������ǻ���̼̼˫�� | |
| B�� | ���ڴ�����������H2�����ӳɷ�Ӧ | |
| C�� | ���л��ﲻ�ܷ����Ӿ۷�Ӧ | |
| D�� | ��ŨH2SO4�����������ᷢ��������Ӧ |