��Ŀ����
��13�֣�(1)1g̼������ˮ������Ӧ����CO��H2��������10.94KJ�������Ȼ�ѧ����ʽΪ
(2)��֪��1��105 Pa��298 K�����£�2 mol����ȼ������Һ̬ˮ�ų�571.6kJ����������ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ��
��3��Na2CO3����Һ���ܴ����ĥ�ڲ��������Լ�ƿ�У���ԭ���ǣ�������ӷ���ʽ˵������ �������Ȼ�����Һ���ɲ��������,���õ��Ĺ�������ǣ���ѧʽ�� ����ط�Ӧ�ķ���ʽ�ǣ� �� ������FeCl2��Һʱ��Ӧ���� ��������Fe2+��ˮ�⣬��Ӧ�����������ۣ�Ŀ���ǣ� ��
(2)��֪��1��105 Pa��298 K�����£�2 mol����ȼ������Һ̬ˮ�ų�571.6kJ����������ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ��
��3��Na2CO3����Һ���ܴ����ĥ�ڲ��������Լ�ƿ�У���ԭ���ǣ�������ӷ���ʽ˵������ �������Ȼ�����Һ���ɲ��������,���õ��Ĺ�������ǣ���ѧʽ�� ����ط�Ӧ�ķ���ʽ�ǣ� �� ������FeCl2��Һʱ��Ӧ���� ��������Fe2+��ˮ�⣬��Ӧ�����������ۣ�Ŀ���ǣ� ��
(1) C(s)+ H2O(g) == CO(g) + H2(g)��H== +131.3KJ��mol��1
(2) H2(g)��O2(g)===H2O(l)����H��-285.6 kJ��mol��1
��3��CO32-��H2O HCO3��+OH-���������˵������Fe2O3��
HCO3��+OH-���������˵������Fe2O3��
Fe3+ + 3H2O Fe��OH��3 + 3H+��2Fe��OH��3=��=Fe2O3+3H2O�� ��� ��ֹFe2+��������
Fe��OH��3 + 3H+��2Fe��OH��3=��=Fe2O3+3H2O�� ��� ��ֹFe2+��������
(2) H2(g)��O2(g)===H2O(l)����H��-285.6 kJ��mol��1
��3��CO32-��H2O
 HCO3��+OH-���������˵������Fe2O3��
HCO3��+OH-���������˵������Fe2O3��Fe3+ + 3H2O
 Fe��OH��3 + 3H+��2Fe��OH��3=��=Fe2O3+3H2O�� ��� ��ֹFe2+��������
Fe��OH��3 + 3H+��2Fe��OH��3=��=Fe2O3+3H2O�� ��� ��ֹFe2+���������Ȼ�ѧ����ʽ����дע�⣺�ٸ����ʺ�������ע���ۼ�״̬
�ڦ�H��ע����+����-������ʾ���Ȼ����
�ۦ�H����ֵ�뻯ѧ��Ӧ����ʽ�Ļ�ѧ����ϵ����Ӧ�����Ȼ�ѧ����ʽ�У���ѧ��Ӧ����ʽ�Ļ�ѧ����ϵ����ʾ�μӷ�Ӧ���ʵ����ʵ���
�ܦ�H�ĵ�λ��KJ��mol��1��J��mol��1
��1�������Ļ��㣺10.94KJ/��1g /12g.mol-1��=131.3KJ��mol��1
(2)ȼ���ȵĶ��壺��25�棬101 kPaʱ��1 mol��ȼ����ȫȼ�������ȶ��Ļ�����ʱ���ų���������
(3)��ΪNa2CO3ˮ��ʹ��Һ�Լ��ԣ���OH-���벣���еĶ������跴Ӧ������ճ�ԵĹ����ƣ�ʹƿ������
���Ȼ�����Һ�У����Ȼ���ˮ�������������������ɣ��ã��������������������, ���������ֽ�����Fe2O3��
�ڦ�H��ע����+����-������ʾ���Ȼ����
�ۦ�H����ֵ�뻯ѧ��Ӧ����ʽ�Ļ�ѧ����ϵ����Ӧ�����Ȼ�ѧ����ʽ�У���ѧ��Ӧ����ʽ�Ļ�ѧ����ϵ����ʾ�μӷ�Ӧ���ʵ����ʵ���
�ܦ�H�ĵ�λ��KJ��mol��1��J��mol��1
��1�������Ļ��㣺10.94KJ/��1g /12g.mol-1��=131.3KJ��mol��1
(2)ȼ���ȵĶ��壺��25�棬101 kPaʱ��1 mol��ȼ����ȫȼ�������ȶ��Ļ�����ʱ���ų���������
(3)��ΪNa2CO3ˮ��ʹ��Һ�Լ��ԣ���OH-���벣���еĶ������跴Ӧ������ճ�ԵĹ����ƣ�ʹƿ������
���Ȼ�����Һ�У����Ȼ���ˮ�������������������ɣ��ã��������������������, ���������ֽ�����Fe2O3��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
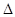 H=��
H=��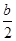 kJ/mol
kJ/mol 2NO(g) DH��a kJ��mol��1��ƽ�ⳣ��K���±���
2NO(g) DH��a kJ��mol��1��ƽ�ⳣ��K���±���