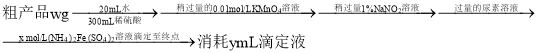��Ŀ����
����Ŀ����ľ����Ҫ�ɷ���̼��ء��ִӲ�ľ������ȡ���Σ�����ʵ��������е�S![]() ��C
��C![]() ��Cl-��
��Cl-��
(1)�Ӳ�ľ������ȡ���ε�ʵ�����˳�����£��ٳ�����Ʒ�����ܽ�ͳ�������______����_______������ȴ�ᾧ��
(2)��������ƽ(ָ�����ϵ�)������Ʒʱ����ָ��ƫ���ұߣ����ʾ___��
A.�����أ���Ʒ�� B.�����ᣬ������
C.�����أ������� D.�����ᣬ��Ʒ��
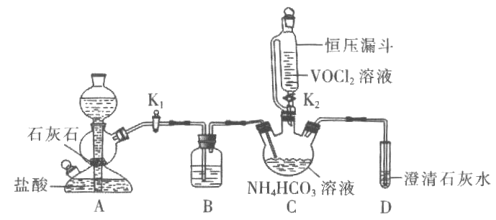
(3)�ڽ��Тڢۢܲ���ʱ��Ҫ�õ��������������÷ֱ���_____��______��______��
(4)���Ƶõ�������������Թܣ�������ˮ�ܽⲢ����Һ�ֳ����ݣ���װ����֧�Թ��
���ڵ�һ֧�Թ������ϡ���ᣬ�ɹ۲���___���ɣ�֤����Һ����_____��
���ڵڶ�֧�Թ����������ϡ������ټ����Ȼ�����Һ���ɹ۲���________���ɣ�֤����Һ����__________��
���ڵ���֧�Թ����������ϡ������ټ�����������Һ���ɹ۲���______���ɣ�֤����Һ����Cl-��
���𰸡����� ���� B �����ܽ� ���� ���Ⱦ��ȣ���ֹҺ��ɽ��� ���� ![]() ��ɫ����
��ɫ���� ![]() ��ɫ����
��ɫ����
��������
��1����ľ���еļ��ο�������ˮ���γ���Һ���������ù��˵ķ�������ü��ε�ˮ��Һ��
��2��������ƽƽ���ԭ�����ش�
��3�����ݲ������������ǽ�����������ش�
��4����̼������ӿ��Ժ����ᷴӦ����ˮ�Ͷ�����̼��
����������ӿ��Ժͱ�����֮�䷴Ӧ���ɲ�����������ᱵ������
�������ӡ�̼��������Լ���������Ӿ����Ժ������ӷ�Ӧ���ɳ�����
(1)��ľ���еļ��ο�������ˮ���γ���Һ��Ȼ�����ù��˵ķ������Ի�ü��ε�ˮ��Һ�������õ�ˮ��Һ�����ᾧ���Ի���Ȼ��صĹ��壻
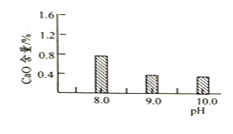
(2) ��������ƽ������Ʒʱ����ָ��ƫ���ұߣ����ʾ�����ᡢ�����أ��ʴ�Ϊ��B��
(3)���ܽ����ʱ��ʹ�ò��������Լ����ܽ⣬�ڹ��˲����У������ò��������������������ᾧ�����У��ò����������裬ʹ�����Ⱦ��ȣ���ֹҺ��ɽ������ʴ�Ϊ�������ܽ⣻���������Ⱦ��ȣ���ֹҺ��ɽ�����
(4)��̼��������ܺ����ᷴӦ���ɶ�����̼������Һ�м���ϡ���������������˵����̼������ӣ��ʴ�Ϊ�����ݣ�CO32-��
����ԭ��Һ�еμ������ữ���Ȼ�����Һ���ų������������ӵĸ��ţ����������ɫ��������˵����Һ������������ӣ��ʴ�Ϊ����ɫ������SO42-��
����ԭ��Һ�еμ�����ϡ������ų����������ӵĸ��ţ��ټ���AgNO3��Һ��������ɫ������˵����Һ���������ӣ��ʴ�Ϊ����ɫ������

 ȫ�ܲ��һ���þ�ϵ�д�
ȫ�ܲ��һ���þ�ϵ�д�����Ŀ����ҵ�ӷ�Ǧ�����ص����ࣨ��Ҫ�ɷ�ΪPbSO4��PbO2������Ǧ��RSR ���յ���Ҫ�������£�
![]()
��1��Ǧ�����طŵ�ʱ�ܷ�ӦΪ��
Pb(s) �� PbO2(s) �� 2H2SO4(aq) �� 2PbSO4(s) �� 2H2O(l)
������Ӧ��PbO2(s) �� SO42��(aq) �� 4H+(aq) �� 2e�� �� PbSO4(s) �� 2H2O(l)
������Ӧ��________��
��2���������м���Na2CO3��Һ��PbSO4ת��Ϊ�����ܵ�PbCO3��
���û�ѧƽ���ƶ�ԭ��������ԭ��________��
�ڹ�ҵ�ϳ���NaHCO3��Һ����Na2CO3��Һ����PbSO4ת��ΪPbCO3��PbSO4��NaHCO3��Һ��Na2CO3��Һ��ͬ���ʵ�����ʱ��PbSO4��ת���ʼ��±���
�� | n(PbSO4)�� n(NaHCO3) | 1��1.5 | 1��2 | 1��3 |
PbSO4ת����/% | 95.5 | 96.9 | 97.8 | |
�� | n(PbSO4)�� n(Na2CO3) | 1��1.5 | 1��2 | 1��3 |
PbSO4ת����/% | 98 | 98 | 98 |
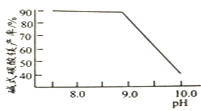
�����ϱ����ݣ����ʵ�������ͬʱ������PbSO4��ת���ʱȢ��е��Դ�ԭ����________��
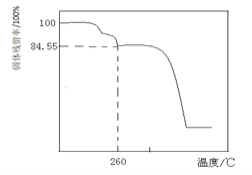
��������Ӧ������PbCO3�⣬���������ɼ�ʽ̼��Ǧ[2PbCO3��Pb(OH)2]���������ȶ��ֽ�����PbO��ͨ��ʵ��ȷ�������к���2PbCO3��Pb(OH)2�����ʵ�������������________��ͨ������ʵ��ȷ��������2PbCO3��Pb(OH)2�ĺ���������ⶨ��������________��
��3�������м���Na2SO3��Һ��������������________��
��4��H2SiF4��Һ�ܽ�PbCO3�Ļ�ѧ����ʽ��________��
����Ŀ��ijʵ���ҷ�Һ��![]() ��Na+��Fe3+��Cr3+��
��Na+��Fe3+��Cr3+��![]() ��
��![]() �����ӣ���ͨ���������̱��Ϊ���Ʊ�K2Cr2O7��
�����ӣ���ͨ���������̱��Ϊ���Ʊ�K2Cr2O7��
��֪��
(a)![]() ��
��![]()
(b)����������������������pH�������
�������� | pH | |
��ʼ���� | ��ȫ���� | |
Fe3+ | 2.7 | 3.7 |
Cr3+ | 4.9 | 6.8 |
��ش�
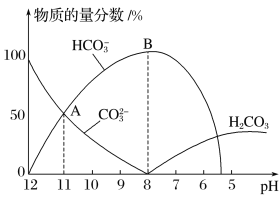
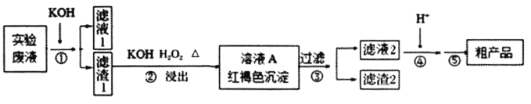
(1)ijͬѧ����ֽ�������жϲ���ټ���KOH�����Ƿ���ʡ��ڼ���һ����KOH��Һ����ëϸ��ȡ��������������ɫ��չ������Ѭ��İߵ���ͼ��ʾ������KOH���ʺϵ�ʵ������(ʵ��˳���Ѵ���)________��C�İߵ���ɫΪ________��
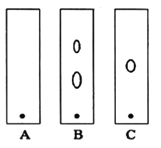
(2)����ں�Cr���ʷ�������Ҫ��Ӧ�����ӷ���ʽΪ________________________��
(3)������װ���У���Ӧѡ��________��(����)
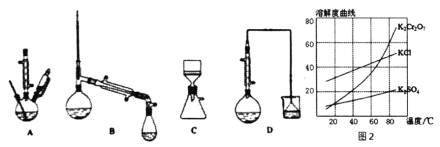
(4)�������ʵ��ܽ��������ͼ2������ݿ����õ����в��ֲ�����a�����������ִ������壬ֹͣ���ȣ�b����ȴ�����£�c����������Һ���־�Ĥ��ֹͣ���ȣ�d��ϴ�ӣ�e�����ȹ��ˣ�f�����ˡ���ѡ����ʲ�������ȷ˳��________��
(5)������к��ʵ�ϴ�Ӽ���________(����ˮ�Ҵ��������Ҵ�-ˮ���Һ��������ˮ��������ˮ��)��
(6)ȡmg�ֲ�Ʒ���250mL��Һ��ȡ25.00mL����ƿ�У���cmol��L-1��(NH4)2Fe(SO4)2����Һ�ζ�(���ʲ���Ӧ)�����ı�(NH4)2Fe(SO4)2��ҺVmL����ôֲ�Ʒ��K2Cr2O7�Ĵ���Ϊ________��