��Ŀ����
����Ŀ������![]() ��ʽ̼���
��ʽ̼���![]() ������ˮ
������ˮ![]() ��ѧʽΪ
��ѧʽΪ![]() �������Ʊ���������
�������Ʊ���������![]() ��ԭ�ϡ�
��ԭ�ϡ�![]() �۷��������������¾��л�ԭ�ԡ�һ���Ʊ�����
�۷��������������¾��л�ԭ�ԡ�һ���Ʊ�����![]() ��ʽ̼��立������£�
��ʽ̼��立������£�
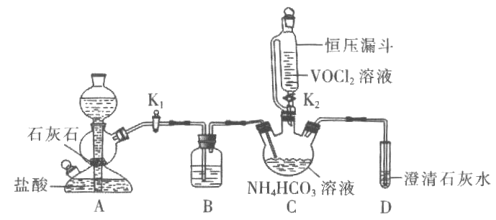
��ش��������⣺
(1)![]() װ��ʢװ���Լ���________
װ��ʢװ���Լ���________![]() ������
������![]() ��A�з�Ӧ�����ӷ���ʽΪ________����Է�Һ©������ѹ©���ŵ���________��
��A�з�Ӧ�����ӷ���ʽΪ________����Է�Һ©������ѹ©���ŵ���________��
(2)ʵ��ʱ���ȹر�![]() ����
����![]() ����________
����________![]() ��ʵ������
��ʵ������![]() ʱ����________
ʱ����________![]() ��ʵ�����
��ʵ�����![]() ��
��
(3)ʵ����Ϻ�Cװ���з����Ʒ�IJ���������________![]() ���������
���������![]() ��
��
(4)�ⶨ�ֲ�Ʒ�з��ĺ�����ʵ�鲽�����£�
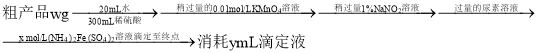
��֪���ζ���ӦΪ![]()
![]() �ôֲ�Ʒ�з������������ı���ʽΪ________
�ôֲ�Ʒ�з������������ı���ʽΪ________![]() ��
��
![]() ���ζ�ǰ���Ӷ������յ�ʱ���Ӷ��������ý��________����ƫ��������ƫ����������Ӱ����
���ζ�ǰ���Ӷ������յ�ʱ���Ӷ��������ý��________����ƫ��������ƫ����������Ӱ����![]() ��
��
���𰸡�����![]() ��Һ
��Һ ![]() ����Һ��˳������
����Һ��˳������ ![]() ����Һ����� �ر�
����Һ����� �ر�![]() ����
����![]() ����
���� ![]() ƫ��
ƫ��
��������
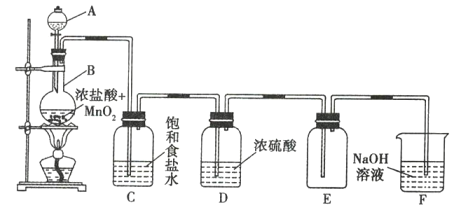
![]() װ����ʵ������ȡ������̼��װ�ã�������̼�л��е�����������Ҫ��HCl����ʵ��IJ�ƷΪ��ʽ̼���Σ�����HCl��Ӧ������Ҫ�ñ���
װ����ʵ������ȡ������̼��װ�ã�������̼�л��е�����������Ҫ��HCl����ʵ��IJ�ƷΪ��ʽ̼���Σ�����HCl��Ӧ������Ҫ�ñ���![]() ��Һ��ȥ
��Һ��ȥ![]() �����л��е�����HCl����ѹ©���ŵ���ƽ������ѹǿ��ʹ©����Һ����˳�����£�
�����л��е�����HCl����ѹ©���ŵ���ƽ������ѹǿ��ʹ©����Һ����˳�����£�
![]() ����ɿ�֪
����ɿ�֪![]() �۷��������������¾��л�ԭ�ԣ��ױ�����������C��ͨ��
�۷��������������¾��л�ԭ�ԣ��ױ�����������C��ͨ��![]() ��Ŀ���dz�ȥ��������ֹ
��Ŀ���dz�ȥ��������ֹ![]() ������Ʒ��Dװ�õij���ʯ��ˮ�������ڼ���
������Ʒ��Dװ�õij���ʯ��ˮ�������ڼ���![]() ����D����Һ�����ʱ������C�п������ž�����ʱ�ر�
����D����Һ�����ʱ������C�п������ž�����ʱ�ر�![]() ����
����![]() ����
����![]() ��Һ���뵽
��Һ���뵽![]() ��Һ�з�����Ӧ��
��Һ�з�����Ӧ��
![]() ����ɿ�֪����
����ɿ�֪����![]() ��ʽ̼���������ˮ����ʵ����Ϻ�Cװ���з����Ʒ�IJ��������ǹ��ˣ�
��ʽ̼���������ˮ����ʵ����Ϻ�Cװ���з����Ʒ�IJ��������ǹ��ˣ�
(4) �ټ���![]() ��Һ������
��Һ������![]() ��ʽ̼�������Ϊ
��ʽ̼�������Ϊ![]() ����
����![]() ��ȥ������
��ȥ������![]() �������س�ȥ������
�������س�ȥ������![]() ��������
��������![]() ��Һ��
��Һ��![]() ������Ӧ��
������Ӧ��![]() ���ɵζ���Ӧʽ֪��
���ɵζ���Ӧʽ֪��![]() mol����ôֲ�Ʒ�з���������������ʼ���Ӷ�����
mol����ôֲ�Ʒ�з���������������ʼ���Ӷ�����![]() ƫ���յ㸩�Ӷ�����
ƫ���յ㸩�Ӷ�����![]() ƫС����
ƫС����![]() ƫС����ý��ƫ�͡�
ƫС����ý��ƫ�͡�
![]() װ����ʵ������ȡ������̼��װ�ã�A�з�Ӧ�����ӷ���ʽΪ��
װ����ʵ������ȡ������̼��װ�ã�A�з�Ӧ�����ӷ���ʽΪ��![]() ��������̼�л��е�����������Ҫ��HCl����ʵ��IJ�ƷΪ��ʽ̼���Σ�����HCl��Ӧ������Ҫ�ñ���
��������̼�л��е�����������Ҫ��HCl����ʵ��IJ�ƷΪ��ʽ̼���Σ�����HCl��Ӧ������Ҫ�ñ���![]() ��Һ��ȥ
��Һ��ȥ![]() �����л��е�����HCl����Է�Һ©������ѹ©���ŵ���ƽ������ѹǿ��ʹ©����Һ����˳�����£�
�����л��е�����HCl����Է�Һ©������ѹ©���ŵ���ƽ������ѹǿ��ʹ©����Һ����˳�����£�
![]() ����ɿ�֪
����ɿ�֪![]() �۷��������������¾��л�ԭ�ԣ��ױ�����������C��ͨ��
�۷��������������¾��л�ԭ�ԣ��ױ�����������C��ͨ��![]() ��Ŀ���dz�ȥ��������ֹ
��Ŀ���dz�ȥ��������ֹ![]() ������Ʒ��Dװ�õij���ʯ��ˮ�������ڼ���
������Ʒ��Dװ�õij���ʯ��ˮ�������ڼ���![]() ����D����Һ�����ʱ������C�п������ž�����ʱ�ر�
����D����Һ�����ʱ������C�п������ž�����ʱ�ر�![]() ����
����![]() ����
����![]() ��Һ���뵽
��Һ���뵽![]() ��Һ�з�����Ӧ��
��Һ�з�����Ӧ��
![]() ����ɿ�֪����
����ɿ�֪����![]() ��ʽ̼���������ˮ����ʵ����Ϻ�Cװ���з����Ʒ�IJ��������ǹ��ˣ�
��ʽ̼���������ˮ����ʵ����Ϻ�Cװ���з����Ʒ�IJ��������ǹ��ˣ�
![]() ����
����![]() ��Һ������
��Һ������![]() ��ʽ̼�������Ϊ
��ʽ̼�������Ϊ![]() ����
����![]() ��ȥ������
��ȥ������![]() �������س�ȥ������
�������س�ȥ������![]() ��������
��������![]() ��Һ��
��Һ��![]() ������Ӧ��
������Ӧ��![]() ���ɵζ���Ӧʽ֪��
���ɵζ���Ӧʽ֪��![]() mol����ôֲ�Ʒ�з�����������
mol����ôֲ�Ʒ�з�����������![]() ��
��
![]() ��ʼ���Ӷ�����
��ʼ���Ӷ�����![]() ƫ���յ㸩�Ӷ�����
ƫ���յ㸩�Ӷ�����![]() ƫС����
ƫС����![]() ƫС����ý��ƫ�͡�
ƫС����ý��ƫ�͡�