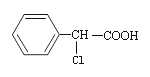
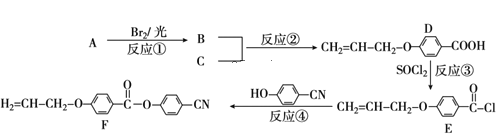
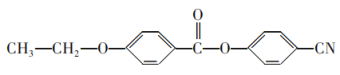
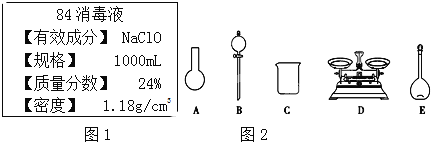
��Ŀ����
����Ŀ��������һ�ַdz���Ҫ�Ļ���ԭ�ϣ��ڲ��������ϡ��ϳ�ϴ�Ӽ��ȹ�ҵ�����Ź㷺��Ӧ�á�
(1)��ҵ���������Ƽ����NaCl��NH3��CO2��ˮ��Ϊԭ���Ʊ�����䷴Ӧԭ��Ϊ��NaCl+NH3+CO2+H2O=NaHCO3��+NH4Cl.
��������Ĺ�������ʾ��ͼ���£�
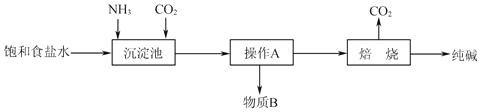
��ش��������⣺
��������NaHCO3�����п��ܺ����������������ʣ�����þ������Ƿ������������ʵIJ�����������__________________��
�ڸù��������пɻ��������õ�������______________________��
(2)��������10ml0.1 mol/L-Na2CO3��Һ����μ���0.1mol/LҺ20mL����Һ�к�̼Ԫ�صĸ��������������������ᣩ����ҺpH�仯�IJ����������ͼ��ʾ��
����ͼʾ�ش���������:
����ͬһ��Һ�У�CO32-��HCO3-��H2CO3________(���������������������������档
���ڵμ�����Ĺ�����HCO3-�����ʵ��������Ӻ���ٵ�ԭ����____________��________________________(��ֱ������ӷ���ʽ��ʾ����
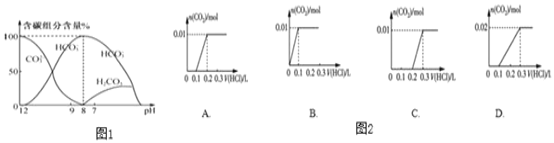
�۽�0.84g NaHCO3��1.06gNa2CO3��ϲ������Һ������Һ�еμ�0.10mol/Lϡ���ᡣͼ������ȷ��ʾ�ӻ�����������������CO2�����ʵ����Ĺ�ϵ����__________������ĸ����
���𰸡�ȡ������������ˮ��������������Һ������ϡ���ᣬ��������ɫ����������Cl����֮��û�� CO2 ���� CO32+H+�THCO3 HCO3+H+�TH2O+CO2�� D��
��������
�����Ƽ���������ڰ������͵��Ȼ�����Һ��ͨCO2���壬��̼�����Ƶ��ܽ�ȱ�̼����С����̼�����Ƴ������ɣ������ˡ�ϴ�Ӹ�����ٽ�̼�����Ƽ��ȷֽ�ɵô��ͬʱ���ɵ�CO2����ѭ�����ã��ݴ˷����ɽ��
(1) ��������NaHCO3�����п��ܺ����������������ʣ�����þ������Ƿ������������ʵIJ�����������ȡ������������ˮ��������������Һ������ϡ���ᣬ��������ɫ����������Cl����֮��û�С�
����ͼ��֪���ù��������пɻ��������õ�������CO2��
(2)��ͼ����֪��̼���̼��������棬
����ͬһ��Һ�У�CO32-��HCO3-��H2CO3���ܴ������档
���ڵμ�����Ĺ�����HCO3-�����ʵ��������Ӻ���ٵ�ԭ��������������Ũ�Ȳ�������CO32+H+�THCO3��HCO3+H+�TH2O+CO2����
��0.84g NaHCO3�����ʵ���Ϊ0.01mol��1.06gNa2CO3�����ʵ���Ϊ0.01mol����ϲ������Һ������Һ�еμ�0.10mol/Lϡ���ᣬ��������̼����1��1��Ӧ��ȫ��ת��Ϊ̼�����ƣ�Ȼ��ȫ��0.02mol̼������������1��1��Ӧ����0.02mol������̼��ͼ������ȷ��ʾ�ӻ�����������������CO2�����ʵ����Ĺ�ϵ����D��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�