ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩΡ≥―ß…ζΉω≈®ΝρΥα–‘÷ ΒΡ Β―ιΘΚ
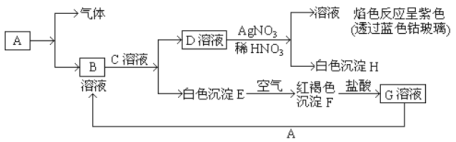
‘Ύ“Μ÷ß ‘Ιή÷–Ζ≈»κ“ΜΩιΚή–ΓΒΡΆ≠Τ§Θ§‘ΌΦ”»κ2mL≈®ΝρΥαΘ§»ΜΚσΑ― ‘ΙήΙΧΕ®‘ΎΧζΦήΧ®…œΓΘΑ―“Μ–ΓΧθ’Κ”–ΤΖΚλ»ή“ΚΒΡ¬Υ÷ΫΖ≈»κ¥χ”–ΒΞΩΉœπΤΛ»ϊΒΡ≤ΘΝßΙή÷–ΓΘ»ϊΫτ ‘ΙήΩΎΘ§‘Ύ≤ΘΝßΙήΩΎ¥Π≤χΖ≈“ΜΆ≈’Κ”–NaOH»ή“ΚΒΡΟόΜ®ΓΘΦ”»» ‘ΙήΘ§Ιέ≤λœ÷œσΓΘ
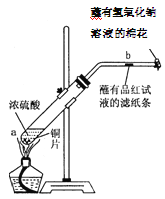
ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©–¥≥ω ‘Ιή÷–ΖΔ…ζΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ____________________ΓΘ
Θ®2Θ© ‘Ιή÷–ΒΡ“ΚΧεΖ¥”Π“ΜΕΈ ±ΦδΚσΘ§b¥Π¬Υ÷ΫΧθΒΡ±δΜ·ΈΣ_______________Θ§¥ΐ ‘Ιή÷–Ζ¥”ΠΆΘ÷ΙΚσΘ§Ηχ≤ΘΝßΙήΖ≈”–’ΚΙΐΤΖΚλ»ή“ΚΒΡ¬Υ÷Ϋ¥ΠΈΔΈΔΦ”»»Θ§¬Υ÷ΫΧθΒΡ±δΜ·ΈΣ_____________ΓΘ
Θ®3Θ©’Κ”–NaOH»ή“ΚΒΡΟόΜ®Ά≈Ής”Ο «________________________________________ΓΘ
Θ®4Θ©ΝρΥα–ΆΥα”ξΒΡ–Έ≥…Ιΐ≥ΧΩ…”Οœ¬Ν–Ζ¥”Π÷–ΒΡ__________ά¥±μ ΨΓΘ
A:SO2+H2O![]() H2SO3
H2SO3
B:O2+2H2SO3=2H2SO4
C:SO2+H2O2=H2SO4
ΓΨ¥πΑΗΓΩCu+2 H2SO4(≈®)![]() CuSO4+ SO2Γϋ+2H2O ’Κ”–ΤΖΚλ»ή“ΚΒΡ¬Υ÷ΫΆ …Ϊ ¬Υ÷Ϋ±δΚλ Έϋ ’Έ¥Ζ¥”ΠΒΡSO2ΤχΧεΘ§Ζά÷ΙΈέ»ΨΩ’Τχ AB
CuSO4+ SO2Γϋ+2H2O ’Κ”–ΤΖΚλ»ή“ΚΒΡ¬Υ÷ΫΆ …Ϊ ¬Υ÷Ϋ±δΚλ Έϋ ’Έ¥Ζ¥”ΠΒΡSO2ΤχΧεΘ§Ζά÷ΙΈέ»ΨΩ’Τχ AB
ΓΨΫβΈωΓΩ
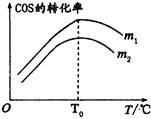
Θ®1Θ©Ά≠ΚΆ≈®ΝρΥα‘ΎΦ”»»ΧθΦΰœ¬ΡήΖΔ…ζΖ¥”ΠΘ§…ζ≥…ΝρΥαΆ≠ΚΆΕΰ―θΜ·ΝρΓΔΥ°ΘΜ
Θ®2Θ©Εΰ―θΜ·ΝρΡή ΙΤΖΚλ»ή“ΚΆ …ΪΘ§ΒΪΕΰ―θΜ·ΝρΒΡΤ·ΑΉ–‘≤ΜΈ»Ε®Θ§Φ”»» ±ΜαΜ÷Η¥‘≠ά¥ΒΡ―’…ΪΘΜ
Θ®3Θ©Εΰ―θΜ·Νρ”–ΕΨΘ§Υυ“‘≤ΜΡή÷±Ϋ”≈≈Ω’Θ§Εΰ―θΜ·Νρ «Υα–‘―θΜ·ΈοΘ§ΡήΚΆΦνΖ¥”Π…ζ≥…―ΈΚΆΥ°ΘΜ
Θ®4Θ©ΝρΥα–ΆΥα”ξΒΡ–Έ≥…Ιΐ≥ΧΘ§άϊ”ΟΗΓ≥ΨΒ»¥ΏΜ·Ής”Οœ¬Θ§―θΤχΑ――«ΝρΥα―θΜ·ΈΣΝρΥαΓΘ
Θ®1Θ©Ά≠ΚΆ≈®ΝρΥα‘ΎΦ”»»ΧθΦΰœ¬ΡήΖΔ…ζΖ¥”ΠΘ§≈®ΝρΥαΨΏ”–«Ω―θΜ·–‘Θ§±ΜΆ≠ΜΙ‘≠ΈΣΕΰ―θΜ·ΝρΘ§Υυ“‘≤ζΈο”–…ζ≥…ΒΡΝρΥαΆ≠ΓΔΕΰ―θΜ·ΝρΚΆΥ°ΘΜ ι–¥Μ·―ßΖΫ≥Χ ΫΈΣCu+2 H2SO4(≈®)![]() CuSO4+ SO2Γϋ+2H2OΘΜ
CuSO4+ SO2Γϋ+2H2OΘΜ
Ι ¥πΑΗΈΣΘΚCu+2 H2SO4(≈®)![]() CuSO4+ SO2Γϋ+2H2OΘΜ
CuSO4+ SO2Γϋ+2H2OΘΜ
Θ®2Θ©Εΰ―θΜ·ΝρΡήΚΆ”–…ΪΈο÷ …ζ≥…Έό…ΪΈο÷ Θ§Εΰ―θΜ·ΝρΡή ΙΤΖΚλ»ή“ΚΆ …ΪΘ§Υυ“‘Εΰ―θΜ·ΝρΨΏ”–Τ·ΑΉ–‘ΘΜΒΪ…ζ≥…ΒΡΈό…ΪΈο÷ ≤ΜΈ»Ε®Θ§Φ”»» ±ΜαΜ÷Η¥‘≠ά¥ΒΡ―’…ΪΘΜΗχ≤ΘΝßΙήΖ≈”–’ΚΙΐΤΖΚλ»ή“ΚΒΡ¬Υ÷Ϋ¥ΠΈΔΈΔΦ”»»Θ§Ά …ΪΒΡ¬Υ÷ΫΧθ”÷±δΚλΘΜ
Ι ¥πΑΗΈΣΘΚΤΖΚλ»ή“ΚΆ …ΪΘΜ¬Υ÷Ϋ±δΚλΘΜ
Θ®3Θ©Εΰ―θΜ·Νρ”–ΕΨΘ§Υυ“‘≤ΜΡή÷±Ϋ”≈≈Ζ≈ΒΫΩ’Τχ÷–Θ§Εΰ―θΜ·Νρ «Υα–‘―θΜ·ΈοΘ§ΡήΚΆΦνΖ¥”Π…ζ≥…―ΈΚΆΥ°Θ§Υυ“‘ ‘ΙήΔρΙήΩΎ»ϊ“ΜΆ≈Ϋΰ”–NaOH»ή“ΚΒΡΟόΜ®ΒΡΉς”Ο «Έϋ ’Έ¥Ζ¥”ΠΒΡSO2ΤχΧεΘ§ΖΔ…ζΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣSO2+2OH-=SO32-+H2OΘΜ
Ι ¥πΑΗΈΣΘΚΈϋ ’Έ¥Ζ¥”ΠΒΡSO2ΤχΧεΘ§Ζά÷ΙΈέ»ΨΩ’ΤχΘΜ
Θ®4Θ©ΝρΥα–ΆΥα”ξΒΡ–Έ≥…Ιΐ≥ΧΘ§άϊ”ΟΗΓ≥ΨΒ»¥ΏΜ·Ής”Οœ¬Θ§―θΤχΑ――«ΝρΥα―θΜ·ΈΣΝρΥαΘ§Υυ“‘ABΖ¥”ΠΖϊΚœΘΜ
Ι ¥πΑΗΈΣΘΚABΓΘ

 ‘ΡΕΝΩλ≥ΒœΒΝ–¥πΑΗ
‘ΡΕΝΩλ≥ΒœΒΝ–¥πΑΗΓΨΧβΡΩΓΩœρΥΡ÷ß ‘Ιή÷–Ζ÷±πΦ”»κ…ΌΝΩ≤ΜΆ§ΒΡΈό…Ϊ»ή“ΚΫχ––»γœ¬≤ΌΉςΘ§Ϋα¬έ’ΐ»ΖΒΡ «
≤ΌΉς | œ÷œσ | Ϋα¬έ | |
A | ΒΈΦ”BaCl2»ή“Κ | …ζ≥…ΑΉ…Ϊ≥ΝΒμ | ‘≠»ή“Κ÷–”– |
B | ΒΈΦ”¬»Υ°ΚΆCCl4Θ§’ώΒ¥Θ§Ψ≤÷Ο | œ¬≤ψ»ή“Κœ‘Ήœ…Ϊ | ‘≠»ή“Κ÷–”–I- |
C | ”ΟΫύΨΜ≤§ΥΩ’Κ»Γ»ή“ΚΫχ––―φ…ΪΖ¥”Π | Μπ―φ≥ ΜΤ…Ϊ | ‘≠»ή“Κ÷–”–Na+ΓΔΈόK+ |
D | ΒΈΦ”œΓNaOH»ή“ΚΘ§ΫΪ Σ»σΚλ…Ϊ ·»ο ‘÷Ϋ÷Ο”Ύ ‘ΙήΩΎ | ‘Ιή≤Μ±δάΕ | ‘≠»ή“Κ÷–Έό |
A.AB.BC.CD.D