��Ŀ����
����Ŀ��ʯ�����ƹ����в�����H2S��һ���ж����壬����Ч������ؽ���������⡣
(1)H2S�ĵ���ʽ��_________��
(2)�ȷֽⷨ����H2S
H2S(g)=H2(g)+S(s) ��H1
��֪��i.2H2S(g)+SO2(g)=3S(s)+2H2O(l) ��H2
ii.S(s)+O2(g)=SO2(g) ��H3
��������H2����H3������H1ʱ������Ҫ����_______��Ӧ����H��
��ֱ�Ӽ��ȷֽ�H2Sת���ʵͣ���ѧ�ҷ���MoS2���Դ�H2S�ֽ���ȡ��������ǣ�����մ�Ĥ����ʹ����ѡ���Է��롣��ѧ��ʹ�ó�����MoS2�Ķ���մ�Ĥװ�ý��з�Ӧ��ԭ����_________��
(3)�绯ѧ������H2S
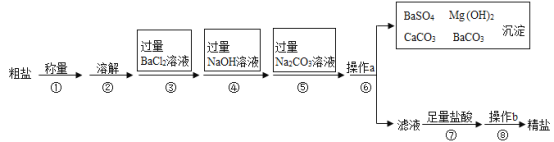
Ϊ������dz��������������������պ͵��������̷ֿ����У�װ����ͼ��ʾ��������������ΪFe2(SO4)3��Һ����ⷴӦ���У���ʯīΪ��������PtΪ�������м������ӽ���Ĥ������
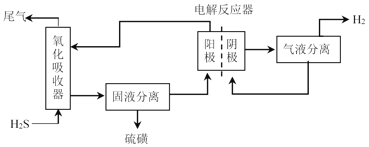
�������������з�Ӧ�����ӷ���ʽΪ_________��
�ڵ�ⷴӦ���У������ĵ缫��ӦʽΪ_________��
�۵�ⷴӦ����������_________(д��2��)��
���𰸡�![]() 2H2(g)+O2(g)=2H2O(l) ʹ��MoS2�ӿ췴Ӧ���ʣ�ʹ�ö���մ�ʹH2����ͨ����H2S(g)
2H2(g)+O2(g)=2H2O(l) ʹ��MoS2�ӿ췴Ӧ���ʣ�ʹ�ö���մ�ʹH2����ͨ����H2S(g)![]() H2(g)+S(s)ƽ�������ƶ����Ӷ����һ��ʱ����H2S��ת���� H2S+2Fe3+=2Fe2++S��+2H+ 2H++2e-=H2�� ����Ƶò���H2��Fe2+-e-=Fe3+��ʵ��Fe2(SO4)3��ѭ��ʹ��
H2(g)+S(s)ƽ�������ƶ����Ӷ����һ��ʱ����H2S��ת���� H2S+2Fe3+=2Fe2++S��+2H+ 2H++2e-=H2�� ����Ƶò���H2��Fe2+-e-=Fe3+��ʵ��Fe2(SO4)3��ѭ��ʹ��
��������
(1)Sԭ����2��Hԭ���γ�2�Թ��õ��Ӷԣ�ʹH2S������ÿ��ԭ�Ӷ��ﵽ�ȶ��ṹ���ݴ���д�����ʽ��
(2)�ٸ��ݸ�˹���ɣ�����֪���Ȼ�ѧ����ʽ���ӣ���֪����Ӧ���Ȼ�ѧ����ʽ��
�ڸ���ƽ���ƶ�ԭ��������
(3)�������������е�Fe2(SO4)3��H2S����Һ�з���������ԭ��Ӧ������FeSO4��H2SO4��S��
���ڵ�ⷴӦ���У��������ҺΪFeSO4��H2SO4����������Һ��H+�õ����ӣ�������ԭ��Ӧ���ݴ���д�缫��Ӧʽ��
�۸���ͼʾ��֪��ȡ�����ʡ��������ѭ��������ⷴӦ�������á�
(1)Sԭ���������6�����ӣ���2��Hԭ���γ�2�Թ��õ��Ӷԣ��Ӷ�ʹH2S������ÿ��ԭ�Ӷ��ﵽ�ȶ��ṹ����H2S�ĵ���ʽΪ��![]() ��
��
(2)��i.2H2S(g)+SO2(g)=3S(s)+2H2O(l) ��H2
ii.S(s)+O2(g)=SO2(g) ��H3
iii. H2S(g)=H2(g)+S(s) ��H1
i+ii-iii��2�������ɵ�2H2(g)+O2(g)=H2O(l)�����Ҫ����H2S(g)=H2(g)+S(s)����H1����Ҫ֪��2H2(g)+O2(g)=H2O(l)�ķ�Ӧ����H��
��ֱ�Ӽ��ȷֽ�H2Sת���ʵͣ� MoS2���Դ�H2S�ֽ���ȡ��������ǣ���ѧ��ʹ�ó�����MoS2�Ķ���մ�Ĥװ�ã�����մ�Ĥ����ʹ����ѡ���Է��룬ʹH2S(g)![]() H2(g)+S(s)���Ӷ�ʹ��ѧƽ�������ƶ������������һ��ʱ����H2S��ת���ʣ�
H2(g)+S(s)���Ӷ�ʹ��ѧƽ�������ƶ������������һ��ʱ����H2S��ת���ʣ�
(3)�������������е�Fe2(SO4)3��H2S����Һ�з���������ԭ��Ӧ������FeSO4��H2SO4��S����Ӧ�����ӷ���ʽΪ��H2S+2Fe3+=2Fe2++S��+2H+��
�ڸ���װ��ͼ��֪�ڵ�ⷴӦ���У��������ҺΪ�����������е�Fe2(SO4)3��H2S��Ӧ�������FeSO4��H2SO4���������ӷŵ�������H+>Fe2+�������ڵ��ص������ϣ���Һ��H+�õ����ӣ�������ԭ��Ӧ���缫��ӦʽΪ��2H++2e-=H2����
���ڵ�ⷴӦ���У������Ϸ�����Ӧ��2H++2e-=H2���������Ϸ�����Ӧ��Fe2+-e-=Fe3+����Ӧ������Fe3+�ٽ�������������������H2S����Ӧ����S���ʣ��Ӷ�ʵ��Fe2(SO4)3��ѭ��ʹ�á�