��Ŀ����
����Ŀ������������ѿ�ӷ���[��Ҫ��Mg(OH)Br���Լ�����NH4Cl�Ͳ�����ˮ���л��ܼ���]��ȡ�������ʵ��������ͼ��
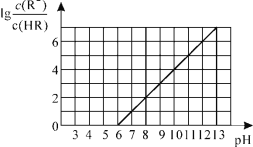
��1����������ʱ��ˮ�������������ԼΪ2��1����ˮ�����˹��ٵ�ԭ����___��
��2��������A����������____��
��3������������Br2ʱ�����ʺϵļ��ȷ�����____��
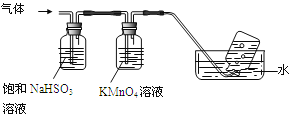
��4����������������װ����ͼ��ʾ��
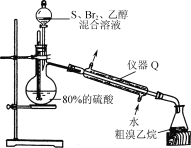
��ͼ������Q��������____��
����ƿ�����������顢����Ļ�ѧ����ʽΪ___��
��5����ƽ�����������д�����ʵ�鷽����___���ô��������������[ʵ���б���ʹ�õ��Լ�������ˮ����ˮCaCl2��1%��NaOH��Һ]��
���𰸡��������ɵ�����þ�ᾧ������Ӱ��������� ��Һ ��ˮԡ ������ 3Br2+S+6C2H5OH![]() 6C2H5Br+2H2O+H2SO4 ��������������1%��NaOH��Һϴ��1��2�Σ���������ˮϴ��2��3�Σ��������ˮCaCl2����
6C2H5Br+2H2O+H2SO4 ��������������1%��NaOH��Һϴ��1��2�Σ���������ˮϴ��2��3�Σ��������ˮCaCl2����
��������
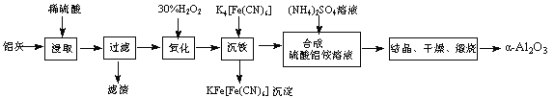
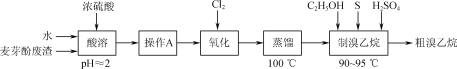
��Ũ�����ܽ�������ѿ�ӷ���[��Ҫ��Mg(OH)Br���Լ�����NH4Cl�Ͳ�����ˮ���л��ܼ���]����������ҺpH=2���ٷ�Һ��ȥ�л��ܼ�������ˮ��Һ��ͨ�����������������Һ�е�Br-����Br2������������������������������£���S��C2H5OH��ϼ����Ƶô������顣
(1)��������ʱ�����ɵ�MgSO4���γɾ��壬���ˮ�����˹��٣�Ŀ���DZ������ɵ�����þ�ᾧ������Ӱ�����������
(2)�л��ܼ���ˮ��Һ�������ܣ��ֲ㣬��ѡ���Һ�������뼴�ɣ�
(3)Br2�ӷ�������ʵ�����̣������¶�Ϊ100��������������Br2ʱֻҪѡ���ˮԡ���ɣ�
(4)��ͼ������Q�������������ܣ�
�������������£�Br2��S��C2H5OH��ϼ����Ƶ������飬Br2����ԭ����S��������������Ӧ�Ļ�ѧ����ʽΪ3Br2+S+6C2H5OH![]() 6C2H5Br+2H2O+H2SO4��
6C2H5Br+2H2O+H2SO4��
(5)���������л����������ˮ�������������д�����ʵ�鷽���ǽ�������������1%��NaOH��Һϴ��1��2�Σ���������ˮϴ��2��3�Σ��������ˮCaCl2���T�ɡ�

 ��ѧ����ͬ����ϰϵ�д�
��ѧ����ͬ����ϰϵ�д�����Ŀ�����ۺ���������ĩ�Ļ�����Ƴɵ����ȼ������ں��Ӹֹ졣��ȡ��ͬ�����ĸ����ȼ��ֱ��100mLͬŨ�ȵ�NaOH��Һ��Ӧ����ȡ���ȼ����������������������ϵ���(����������ڱ�״���²ⶨ)��
�� | �� | �� | |
���ȼ�����/g | 7.5 | 15.0 | 20.0 |
�������/L | 3.36 | 6.72 | 6.72 |
���㣺
��1��NaOH��Һ���ʵ���Ũ��_______
��2�������ȼ���������������_______
��3��15g�����ȼ��������ȷ�Ӧ�����ϲ�������������_______