��Ŀ����
����Ŀ����ұ���ķ���������Ϊԭ����ȡ��ϸ��-���������Ƚ��ͻ�����Ⱦ�ֿ��������Դ�������ʡ���֪���ҵ���Ҫ�ɷ�ΪAl2O3������������SiO2��FeO��Fe2O3�������Ʊ�ʵ���������£�
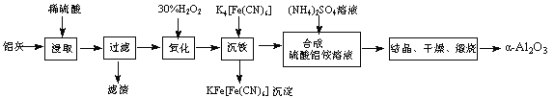
��1�������������������ᷴӦ�Ļ�ѧ����ʽΪ ��
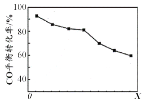
��2��ͼ��������������Ҫ�ɷ�Ϊ (�ѧʽ)��
��3����30%��H2O2��Һ���������ӷ�Ӧ����ʽΪ ��
��4������������茶��壬��������Ҫ��ӦΪ��
4[NH4Al(SO4)2��12H2O]![]() 2Al2O3+ 2NH3��+ N2��+ 5SO3��+ 3SO2��+ 53H2O,������������ͨ����ͼ��ʾ��װ�á�
2Al2O3+ 2NH3��+ N2��+ 5SO3��+ 3SO2��+ 53H2O,������������ͨ����ͼ��ʾ��װ�á�
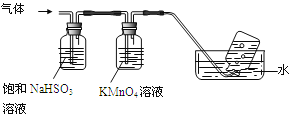
������ƿ���ռ����������� (�ѧʽ)��
����������NaHSO3��Һ���յ����ʳ���H2O��g����� (�ѧʽ)��
��KMnO4��Һ��ɫ��MnO4����ԭΪMn2+�������������ӷ�Ӧ����ʽΪ ��
���𰸡���1��Al2O3+ 3H2SO4= Al2(SO4)3+ 3H2O��2�֣�
��2��SiO2��2�֣�
��3��2Fe2++H2O2+2H+= 2Fe3++2H2O��2�֣�
��4����N2��2�֣���SO3��NH3��2�֣�ȱ©�����֣���
��2MnO4�� +5SO2+ 2H2O = 2Mn2++ 5SO42��+4H+��2�֣�
��������
�������⣬���ҵ���Ҫ�ɷ�ΪAl2O3������������SiO2��FeO��Fe2O3���������м�ϡ���ᣬAl2O3��FeO��Fe2O3ת��Ϊ���ӣ�SiO2���������ᣬ���ˣ���Һ�к���Al3+��Fe2+��Fe3+������˫��ˮ��Fe2+������ΪFe3+������K4[Fe��CN��6]��Fe3+ת��Ϊ���������ˣ�����Һ�м�������泥�����NH4Al��SO4��2���ᾧ��������յõ���-Al2O3��
��1��Al2O3�����ᷴӦ������������ˮ���䷴Ӧ�ķ���ʽΪAl2O3+3H2SO4=Al2(SO4)3+3H2O��
��2����������������ͼ��������������Ҫ�ɷ��Dz���������Ķ������裬��ѧʽΪSiO2��
��3����Һ�к���Al3+��Fe2+��Fe3+����30%��H2O2��Һ��Fe2+������ΪFe3+�����ݵ�ʧ�����غ㡢����غ��ԭ���غ���ƽ��������Ӧ�����ӷ���ʽΪ2Fe2++ H2O2+2H+=2Fe3++2H2O
��4����NH4Al��SO4��212H2O�ֽ����ɵ�����NH3��SO3�������������գ�����������������գ����������ƿ���ռ�����������N2��
������NaHSO3����SO3��������Ӧ������������NaHSO3��Һ���յ����ʳ���H2O��g���⣬����SO3��NH3 ��
�����������£�KMnO4���������Ӧ������������Ӻ������ӣ����ݵ�ʧ�����غ㡢����غ㡢ԭ���غ���ƽ���䷴Ӧ�����ӷ���ʽΪ��2MnO4-+5SO2+2H2O=2Mn2++5SO42-+4H+��

 ����ʦ���һ��һ��ϵ�д�
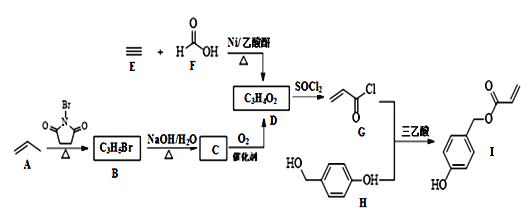
����ʦ���һ��һ��ϵ�д�����Ŀ���Ȼ�������SOCl2��������ҽҩ��ũҩ��Ⱦ�Ϲ�ҵ��Ҳ�����л��ϳɹ�ҵ�����Ȼ�������֪��SOCl2������������±���ʾ��
��ɫ��״̬ | �۵� | �е� | ��ʴ�� | ˮ�� |
��ɫ����Һ�� | -105�� | 78�� | ǿ | ����ˮ�� |
��������ͼװ���Ʊ�SOCl2��
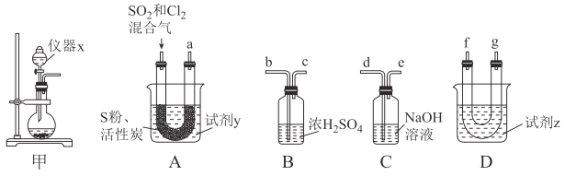
��ش��������⣺
���Ʊ�SO2��Cl2��
��1����ʵ��ѡ��װ�ü��Ʊ�SO2��Cl2��װ�ü�������x������Ϊ___������KMnO4��Ũ���ᷴӦ�Ʊ�Cl2����Ӧ�����ӷ���ʽΪ___��
���Ʊ�SOCl2��
�Ի���̿��Ϊ������SO2��C12���Ժ�S����180~200��ʱ��Ӧ�ϳ�SOCl2��ѡ��װ��A��B��C��D�����Ʊ����г֡�����װ����ȥ����
��2�������������ҵķ���װ��A��B��C��D������˳��Ϊ___���������ӿڵ���ĸ��ţ���
��3���Լ�yΪ___����ѡ����ĸ����ͬ�����Լ�zΪ___��
A����ˮ B���Ҵ� C��ʯ���� D����ˮ
��4��װ��A��U�ι��ڷ�����Ӧ�Ļ�ѧ����ʽΪ___��
��5��װ��C������Ϊ___����װ��A��ͨ���SO2��Cl2�����ʵ���֮��Ϊ1��3����װ��C�����ɵ���Ϊ___���ѧʽ���������ʵ����֤װ��C�����ɵ����к���SO42-��____��
����Ŀ������ͼʾ��������������ϵ���
A | B |
|
|
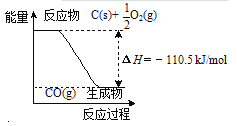
12 g C(s)��һ����O2(g)��Ӧ����14 g CO(g)���ų�������Ϊ110.5 kJ | ��ˮԡ����ƿ��������ɫ��dz |
C | D |
|
|
��֤AgCl�ܽ�ȴ���Ag2S | ��բ����Ϊ�������ܵ����� |
A. AB. BC. CD. D