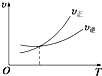
��Ŀ����
9����1��ȡa molA��b mol B����V L�ܱ������ڣ�������Ӧa A��g��+bB��g���TcC��g��+dD��g����1min ����������A ��Ũ�ȼ���x mol/L����ʱB��Ũ��Ϊ$\frac{b}{V}-\frac{bx}{a}$mol/L��D��Ũ��Ϊ$\frac{dx}{a}$molmol/L�����ʱ���ڷ�Ӧ��ƽ��������������A��Ũ�ȱ仯����ʾ��ӦΪxmol/��L•min������2���������ͣ�C3H5N3O9���ֽ�ʱ����ΪN2��CO2��O2��Һ̬ˮ�����ķֽⷴӦ�Ļ�ѧ����ʽ��4C3H5N3O9$\frac{\underline{\;\;��\;\;}}{\;}$6N2+12CO2+O2+10H2O����֪20��ʱ��22.7g�������ͷֽ�ų�������Ϊ154KJ����ÿ����1molҺ̬ˮ����ų�������Ϊ324.2KJ��
���� ��1������c=$\frac{n}{V}$�����A��B��Ũ�ȣ�Ȼ����ݷ�Ӧ����ʽ��A��Ũ�ȱ仯��������ĵ�B������D��Ũ�ȣ�����v=$\frac{��c}{��t}$=����A�Ļ�ѧ��Ӧ���ʣ�
��2���������ͣ�C3H5N3O9���ֽ�ʱ����ΪN2��CO2��O2��Һ̬ˮ�����ù۲취��ƽ����ʽ�����ݷ���ʽ��֪��ÿĦ���������ͷֽ���������壬�ɴ˼����0.1mol�������ͷֽ�������壬������֪�����������1mol����ų���������
��� �⣺��1����ʼʱ��A�����ʵ���Ũ��Ϊ��$\frac{amol}{VL}$=$\frac{a}{V}$mol•L-1��B��Ũ��Ϊ��$\frac{bmol}{VL}$=$\frac{b}{V}$mol/L��
1min ����������A ��Ũ�ȼ���x mol/L�������ʱ���ڷ�Ӧ��ƽ��������������A��Ũ�ȱ仯����ʾΪ��v��A��=$\frac{xmol/L}{1min}$=xmol/��L•min��
��
���ݷ�Ӧa A��g��+bB��g���TcC��g��+dD��g����֪����Ӧ����B��Ũ��Ϊ��xmol/L��$\frac{b}{a}$����1min��B��Ũ��Ϊ��$\frac{b}{V}$mol/L-xmol/L��$\frac{b}{a}$=��$\frac{b}{V}-\frac{bx}{a}$��mol/L������D��Ũ��Ϊ��xmol/L��$\frac{d}{a}$=$\frac{dx}{a}$mol/L��
�ʴ�Ϊ��$\frac{b}{V}-\frac{bx}{a}$��$\frac{dx}{a}$��xmol/��L•min����
��2���������ͣ�C3H5N3O9���ֽ�ʱ����ΪN2��CO2��O2��Һ̬ˮ���������͵�ϵ������Ϊ2����ˮ��ϵ��Ϊ6��������̼�ﵽϵ��Ϊ3������Oԭ��Ϊ1��������ϵ��Ϊ0.5�������������͵�ϵ��Ӧ��Ϊ4��Ȼ�����ù۲취��ƽ�ɵø÷�Ӧ�Ļ�ѧ����ʽΪ��4C3H5N3O9$\frac{\underline{\;\;��\;\;}}{\;}$6N2+12CO2+O2+10H2O��
�������͵�Ħ������Ϊ227g/mol��22.7g�������͵����ʵ���Ϊ��$\frac{22.7g}{227g/mol}$=0.1mol��������������ʵ���Ϊ��$\frac{19}{4}$��0.1mol=0.475mol���ų�154kJ������
������1mol����ų�������Ϊ��$\frac{154kJ}{0.475mol}$=324.2kJ��
�ʴ�Ϊ��4C3H5N3O9$\frac{\underline{\;\;��\;\;}}{\;}$6N2+12CO2+O2+10H2O��324.2��
���� ���⿼���˻�ѧƽ��ļ��㣬��Ŀ�Ѷ��еȣ��漰��ѧ��Ӧ���ʵļ��㡢�Ȼ�ѧ����ʽ�ļ��㡢��ѧ����ʽ����д��֪ʶ����Ŀ�Ѷ��еȣ�����������ϴ�֪ʶ��϶࣬��ֿ���ѧ���ķ�����������ѧ����������ע�����ջ�ѧƽ�⡢��ѧ��Ӧ���ʵĸ�����㷽����

 ij��ѧ��ȤС���ͬѧ������ͼ��ʾʵ��װ�ý���ʵ�飨ͼ��a��b��c��ʾֹˮ�У���
ij��ѧ��ȤС���ͬѧ������ͼ��ʾʵ��װ�ý���ʵ�飨ͼ��a��b��c��ʾֹˮ�У����밴Ҫ����գ�
��1��A��C��E�������װ�ÿ�������ȡCl2��������ص�����ʵ�飮
�����ڱ��м�������ˮ�������Ƶ���ˮ����������ˮ�ֳ����ݣ����Т�����ʵ�飬ʵ����������������£��뽫������ʵ��1�����䣺
| ʵ����� | ʵ����� | ���� | ���� |
| �� | ����ˮ����Ʒ����Һ | ��ɫ | ������ˮ��Ӧ�IJ�����Ư���� |
| �� | ��ˮ�м���NaHCO3��ĩ | ����ɫ���ݲ��� | ������ˮ��Ӧ�IJ������ ��ǿ������ |
����Ҫ��������װ�����һ����ʵ�飬��֤Cl-��Br-�Ļ�ԭ��ǿ�����������ʢװ���Լ�ΪNaBr��Һ���ܵõ����۵�ʵ������Ϊ�Թ�����Һ����ɫ��Ϊ��ɫ��
��ijͬѧ�ø�װ��̽��������KI�ķ�Ӧ���ڱ���ʢ����KI������Һ��ͨ������������������Һ�����ɫ������ͨ������������ɫ����ʧ������Һ���к�+5��Ԫ�ص�������ӣ�����ɫ��ʧ�����б��з�����Ӧ�Ļ�ѧ����ʽ��5Cl2+I2+6H2O=2HIO3+10HCl��
��2��B��D��Eװ����������B��ʢװͭƬ�������п����ϰ��ϣ���Ũ���ᣬ�ر�c����a��b���������Թܶ����ռ���NO2��
��B�з�����Ӧ�Ļ�ѧ����ʽΪCu+4HNO3��Ũ��=Cu��NO3��2+2NO2��+2H2O��
������Dװ����֤NO2��ˮ�ķ�Ӧ�����Թ����ռ���NO2��ʹ�ձ��е�ˮ�����Թܶ��IJ����ǣ����ɸı��Թܺ��ձ���λ�ã����ȹر�ֹˮ�� ab���ٴ�ֹˮ�� c��˫�ֽ��գ����ȣ��Թܶ�ʹ�Թ��������ݳ�������������ˮ�Ӵ��������ձ��е�ˮ�������Թܶ��У�
| A�� | ����ˮ������ղ����ܷ���������Ӧ | |
| B�� | ��ά���������Ƿ������϶��ɣ��ܱ��ֳ���Ԫ�������� | |
| C�� | �����ʵ�ˮ��Һ����ͭ�Σ����Է������� | |
| D�� | ��Ȼ������ˮ�����ղ��ﶼ�Ǧ�-������ |
��������
| A�� | KMnO4 | B�� | Al2��SO4��3 | C�� | KClO3 | D�� | K2HPO4 |
| A�� | ��������ϡNaOH��Һ | B�� | ��������CH3COONa���� | ||
| C�� | ��������NH4HSO4���� | D�� | ��������CuSO4��Һ |
| A�� | ����ˮ | B�� | �������ˮ | C�� | �������ˮ | D�� | ������Ҵ� |
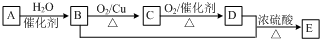
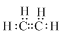 ��������A������ѡ����ˮ�����Ը��������Һ���Լ���
��������A������ѡ����ˮ�����Ը��������Һ���Լ���