��Ŀ����
����Ŀ���о���Ӧ���̵������仯����������������Ҫ���塣
��.�״�����Ҫ�Ļ���ԭ�ϡ����úϳ���(��Ҫ�ɷ�ΪCO��CO2��H2)��һ�������ºϳɼ״����밴Ҫ��ش��������⣺
(1)��ӦCO2(g)��3H2(g) ![]() CH3OH(g)��H2O(g)��ʹ�ú�δʹ�ô���ʱ����Ӧ���̺������Ķ�Ӧ��ϵ��ͼ��ʾ������˵����ȷ����___(����ĸ����)��
CH3OH(g)��H2O(g)��ʹ�ú�δʹ�ô���ʱ����Ӧ���̺������Ķ�Ӧ��ϵ��ͼ��ʾ������˵����ȷ����___(����ĸ����)��
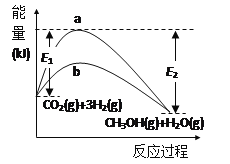
A���ÿ��淴Ӧ������ӦΪ���ȷ�Ӧ
B�����ѷ�Ӧ���еĻ�ѧ�������յ�������С���γ��������еĻ�ѧ�����ͷŵ�������
C������b��ʹ���˴�����Ӧ�����ߣ��Ҽ��������Ӧ�ȱ�С
(2)CO��CO2��H2�ںϳɹ����з�������Ҫ��Ӧ���£�
��CO(g)��2H2(g) ![]() CH3OH(g)����H1����99 kJ��mol��1
CH3OH(g)����H1����99 kJ��mol��1
��CO2(g)��3H2(g) ![]() CH3OH(g)��H2O(g)����H2����58 kJ��mol��1
CH3OH(g)��H2O(g)����H2����58 kJ��mol��1
��CO2(g)��H2(g) ![]() CO(g)��H2O(g)����H3
CO(g)��H2O(g)����H3
����H3��____��
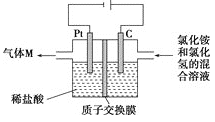
(3)ֱ�Ӽ״�ȼ�ϵ��(���DMFC)������ṹ������ת���ʸߡ��Ի�������Ⱦ������Ϊ������Դ�����Ʒ��Խ��Խ�ܵ���ע��DMFC�Ĺ���ԭ����ͼ��ʾ��
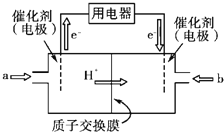
��ͨ��a����ĵ缫�ǵ�ص�____(����������������)������缫��ӦʽΪ___��
��.��֪��������ͨ����������S8(б����)����ʽ���ڣ���������״̬�£���S2��S4��S6��S8�ȶ���ͬ�������壬����S4��S6��S8�������ƵĽṹ�ص㣬��ṹ��ͼ��ʾ��
![]()
(1)��һ���¶��£������������ƽ��Ħ������Ϊ80 g��mol��1�����������S2�����������С��_____��
(2)����֪�������ļ���Ϊd kJ��mol��1���������ļ���Ϊe kJ��mol��1��S(s)��O2(g)===SO2(g)��H����a kJ��mol��1����S8��������ļ���Ϊ_____��
���𰸡�B 41 kJ��mol��1 �� CH3OH��6e����H2O===CO2����6H�� 75% (2d��a��e) kJ��mol��1
��������
I��(1) A���ÿ��淴Ӧ�ķ�Ӧ����������������������������������ӦΪ���ȷ�Ӧ��A����
B������ͼ���֪�����ѷ�Ӧ���еĻ�ѧ�������յ�������(E1)С���γ��������еĻ�ѧ�����ͷŵ�������(E2)��B��ȷ��
C������b�����˷�Ӧ�Ļ����ʹ���˴�����Ӧ�����ߣ��Ҽ��������Ӧ�Ȳ��䣬C����
��ΪB��
(2)���ݸ�˹���ɣ���-�ٿɵ�CO2(g)��H2(g) ![]() CO(g)��H2O(g)����H3=-58+99=+41 kJ��mol��1��
CO(g)��H2O(g)����H3=-58+99=+41 kJ��mol��1��
(3)����ͼ����ת�Ƴ���һ��Ϊ��������aΪ�״����״���ˮ��Ӧ��ʧ�������ɶ�����̼�������ӣ��缫��ӦʽΪCH3OH��6e����H2O==CO2����6H����
II��(1) ��S2��S4ʱ��S2���ӵ����������С����S2���ʵ���ΪX��S4���ʵ���ΪY��![]() =80���õ�X��Y=3��1����ͬ�������������ʵ���֮�ȵ������֮����õ�����������S2���ӵ����������С��75%��
=80���õ�X��Y=3��1����ͬ�������������ʵ���֮�ȵ������֮����õ�����������S2���ӵ����������С��75%��
(2)��ѧ��Ӧ�У��ɼ��Ķ������ȣ��¼����γɷ��ȣ�����H��S-S+e-2d��S8��������ļ���=(2d��a��e) kJ��mol��1��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ������������������ NO �ķ�ӦΪ��N2(g) + O2(g)![]() 2NO(g)��t��ʱ��K= 0.09���� t���¼ס��ҡ������������ܱ������У�Ͷ�� N2(g)�� O2(g)ģ�ⷴӦ����ʼŨ�����±���ʾ��
2NO(g)��t��ʱ��K= 0.09���� t���¼ס��ҡ������������ܱ������У�Ͷ�� N2(g)�� O2(g)ģ�ⷴӦ����ʼŨ�����±���ʾ��
��ʼŨ�� | �� | �� | �� |
c(N2)/mol��L-1 | 0.46 | 0.46 | 0.92 |
c(O2)/mol��L-1 | 0.46 | 0.23 | 0.92 |
�����жϲ���ȷ����
A.��ʼʱ����Ӧ���ʣ������ף���B.ƽ��ʱ��N2 ��ת���ʣ��ף���
C.ƽ��ʱ��c(NO)���ף�������D.ƽ��ʱ������ c(N2)= 0.4mol��L-1