��Ŀ����
����Ŀ�������£�NCl3��һ�ֻ�ɫ��״Һ�壬���Ʊ�����ˮ������ClO2��ԭ�ϣ����Բ�����ͼ��ʾװ���Ʊ�NCl3������˵����ȷ����( )

A.ÿ����1 mol NCl3����������4 mol H�������ӽ���Ĥ���Ҳ������Ǩ��
B.����ʪ���KI��������ֽ��������M
C.ʯī���ĵ缫��ӦʽΪNH4+��3Cl����6e��===NCl3��4H��
D.�������У����ӵ���������Ϊ��Դ������������ʯī������Դ����
���𰸡�C
��������
A. ����ͼ���֪��ʯī�缫���������õ缫�Ϸ���ʧ���ӵ�������ӦNH4++3Cl6e=NCl3+4H+��ÿ����1molNCl3����������6molH+�����ӽ���Ĥ���Ҳ������Ǩ�ƣ�A����
B. Pt�����������������������ӵõ��ӵĻ�ԭ��Ӧ,�缫��ӦʽΪ��2H++2e=H2����������ʪ��ĵ���ֽ�ʼ���������B����
C. ʯī�缫���������õ缫�Ϸ���ʧ���ӵ�������ӦNH4+��3Cl����6e��==NCl3��4H����C��ȷ��
D. �������У����ӽ���Ĥ�Ҳ���Һ�з�����Ӧ��NH4+��3Cl����6e��==NCl3��4H�������Dz����������ӻ���������Pt�缫���Ҳ���Һ��pH�������䣬D����
��ΪC��

����Ŀ��![]() ��
��![]() ��
��![]() ��
��![]() ����Ԫ�أ�ǰ����Ԫ�صļ����Ӷ�����ԭ�Ӿ�����ͬ�ĺ�������Ų���
����Ԫ�أ�ǰ����Ԫ�صļ����Ӷ�����ԭ�Ӿ�����ͬ�ĺ�������Ų���![]() Ԫ��û�������ϼۣ�
Ԫ��û�������ϼۣ�![]() ���⻯��ķ���ʽΪ
���⻯��ķ���ʽΪ![]() ��
��![]() ��
��![]() �ĵ����ܴ���������Һ���û���
�ĵ����ܴ���������Һ���û���![]() ����״������
����״������![]() ��ԭ�Ӻ���û�����ӡ�
��ԭ�Ӻ���û�����ӡ�
��1�����������������ƶ�![]() ��
��![]() ��
��![]() ��
��![]() ��Ԫ�����ơ�
��Ԫ�����ơ�
A________��B ________��C ________��D ________��
��2���õ���ʽ��ʾ![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() ��
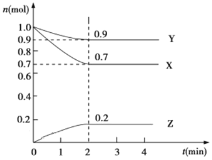
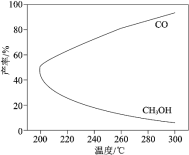
��![]() ���ϳɵĻ����ָ���仯��������ͼ�������ѧ�����͡�
���ϳɵĻ����ָ���仯��������ͼ�������ѧ�����͡�
������ | ����ʽ | ���������� | ��ѧ������ |
| _____ | _____ | _____ |
| _____ | _____ | _____ |
B+D | ____ | _____ | _____ |
��3���![]() ��
��![]() ���γɵĻ������
���γɵĻ������![]() ��
��![]() ���γɵĻ����ﷴӦ�����ӷ���ʽ��_______��
���γɵĻ����ﷴӦ�����ӷ���ʽ��_______��