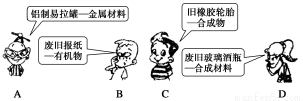
��Ŀ����
��1�������������ʣ�
aNaCl������bҺ̬SO3��cҺ̬�Ĵ�����d����eBaSO4������f��������g�ƾ���h�ۻ���KNO3
��ش���������(��д��Ӧ����ĸ)��
�������������ܵ������________��
���������������ڵ���ʵ���____________��
���������������ڷǵ���ʵ���____________��
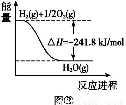
�����Ϲ��ۻ�����������ˮ���γɵ�ˮ��Һ�ܵ������______________��
��2�����й���������ĸ���������ȷ����__________(��д���)��
������������϶��Ƿǽ�����������ǽ���������϶������������������������϶��ǽ�������������������ﶼ�Ǽ�������������������������ˮ��Ӧ������Ӧ���ᡡ����ˮ��Ӧ������������ﲻһ������������ˮ��Ӧ���ɼ�������ﲻһ���Ǽ�������������ܸ��ᷴӦ��������һ���ܸ��Ӧ
��1����dh����aceh����bfg����bc����2���ۢ�
����������2��������������Ҳ�н����������Mn2O7������ȷ�����ǽ�����������ܲ��������������CO������ȷ������ȷ������������������������������Mn2O7������ȷ��������������������費����ˮ��Ӧ������Ӧ�Ĺ��ᣬ����ȷ������ˮ��Ӧ������������ﲻһ������������NO2����ˮ��Ӧ���ɼ�������ﲻһ���Ǽ����������Na2O2������ȷ�������ܸ��ᷴӦ�������Ҳ���ܲ�����Ӧ����CO��NO�ȡ�

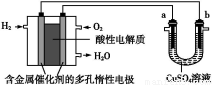
(1)�µġ�����������������(GB 3095 2012)����2016��1��1�����ҹ�ȫ��ʵʩ���ݴ�,������������ָ��(AQI)�ձ���ʵʱ���������SO2��NO2��CO��O3��PM10��PM2.5��ָ��,Ϊ�����ṩ����ָ��,�������ؾ���������ų��к����
2012)����2016��1��1�����ҹ�ȫ��ʵʩ���ݴ�,������������ָ��(AQI)�ձ���ʵʱ���������SO2��NO2��CO��O3��PM10��PM2.5��ָ��,Ϊ�����ṩ����ָ��,�������ؾ���������ų��к����
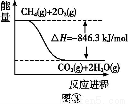
�������ų���β���к���CO��NO������,�û�ѧ����ʽ���Ͳ���NO��ԭ�� ��
�������������ڰ�װ�Ĵ�ת����,��ʹ����β���е���Ҫ��Ⱦ��ת��Ϊ���Ĵ���ѭ�����ʡ���֪:
N2(g)+O2(g)=2NO(g) ��H=+180.5 kJ/mol
2C(s)+O2(g)=2CO(g) ��H=-221.0 kJ/mol
C(s)+O2(g)=CO2(g) ��H=-393.5 kJ/mol
��Ӧ2NO(g)+2CO(g)=N2(g)+2CO2(g)�Ħ�H= kJ/mol��
(2)ֱ���ŷŵ���������γ����ꡢ����,����ԭ�����������շ��dz��õĴ�������������NH3��CH4�������ȥ�����еĵ��������֪:CH4(g)+2O2(g)=CO2(g)+2H2O(l) ��H1=a kJ/mol;�����㷴ӦCH4(g)+4NO(g)=CO2(g)+2H2O(l)+2N2(g)���ʱ䦤H2����Ҫ��ѯij��Ӧ���ʱ䦤H3,����Ӧ�и����ʵĻ�ѧ������֮��Ϊ���������ʱ,��H3=b kJ/mol,�÷�Ӧ���Ȼ�ѧ����ʽ�� ,�ݴ˼������H2= kJ/mol(�ú�a��b��ʽ�ӱ�ʾ)��
(3)�±��г��˹�ҵ������SO2�����ַ�����
������ | �ð�ˮ��SO2ת��(NH4)2SO3,��������(NH4)2SO4 |
������ | ���������Ƚ���(��Ҫ�ɷ�CO��CH4��H2)��SO2�ڸ����»�ԭ�ɵ����� |
������ | ��Na2SO3��Һ����SO2,�پ����ת��ΪH2SO4 |
��������Ҫ���������з�Ӧ:
2CO(g)+SO2(g)=S(g)+2CO2(g) ��H=+8.0 kJ/mol
2H2(g)+SO2(g)=S(g)+2H2O(g)��H=+90.4 kJ/mol
2CO(g)+O2(g)=2CO2(g) ��H=-566.0 kJ/mol
2H2(g)+O2(g)=2H2O(g) ��H=-483.6 kJ/mol
��S(g)��O2(g)��Ӧ����SO2(g)���Ȼ�ѧ����ʽ�ɱ�ʾΪ ��
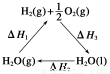
(4)�ϳɰ��õ��������Լ���Ϊԭ���Ƶá��йػ�ѧ��Ӧ�������仯��ͼ��ʾ,��CH4(g)��H2O(g)��Ӧ����CO(g)��H2(g)���Ȼ�ѧ����ʽΪ ��