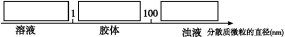
ΧβΡΩΡΎ»ί
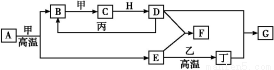
(1)–¬ΒΡΓΕΜΖΨ≥Ω’Τχ÷ ΝΩ±ξΉΦΓΖ(GB 3095 2012)ΫΪ”Ύ2016Ρξ1‘¬1»’‘ΎΈ“Ιζ»ΪΟφ Β ©ΓΘΨί¥Υ,ΜΖΨ≥Ω’Τχ÷ ΝΩ÷Η ΐ(AQI)»’±®ΚΆ Β ±±®ΗφΑϋά®ΝΥSO2ΓΔNO2ΓΔCOΓΔO3ΓΔPM10ΓΔPM2.5Β»÷Η±ξ,ΈΣΙΪ÷ΎΧαΙ©ΫΓΩΒ÷Η“ΐ,“ΐΒΦΒ±ΒΊΨ”ΟώΚœάμΑ≤≈≈≥ω––ΚΆ…ζΜνΓΘ
2012)ΫΪ”Ύ2016Ρξ1‘¬1»’‘ΎΈ“Ιζ»ΪΟφ Β ©ΓΘΨί¥Υ,ΜΖΨ≥Ω’Τχ÷ ΝΩ÷Η ΐ(AQI)»’±®ΚΆ Β ±±®ΗφΑϋά®ΝΥSO2ΓΔNO2ΓΔCOΓΔO3ΓΔPM10ΓΔPM2.5Β»÷Η±ξ,ΈΣΙΪ÷ΎΧαΙ©ΫΓΩΒ÷Η“ΐ,“ΐΒΦΒ±ΒΊΨ”ΟώΚœάμΑ≤≈≈≥ω––ΚΆ…ζΜνΓΘ
ΔΌΤϊ≥Β≈≈≥ωΒΡΈ≤Τχ÷–Κ§”–COΚΆNOΒ»ΤχΧε,”ΟΜ·―ßΖΫ≥Χ ΫΫβ Ά≤ζ…ζNOΒΡ‘≠“ρ ΓΘ
ΔΎΤϊ≥Β≈≈ΤχΙήΡΎΑ≤ΉΑΒΡ¥ΏΜ·ΉΣΜ·Τς,Ω… ΙΤϊ≥ΒΈ≤Τχ÷–ΒΡ÷ς“ΣΈέ»ΨΈοΉΣΜ·ΈΣΈόΕΨΒΡ¥σΤχ―≠ΜΖΈο÷ ΓΘ“―÷Σ:
N2(g)+O2(g)=2NO(g) ΠΛH=+180.5 kJ/mol
2C(s)+O2(g)=2CO(g) ΠΛH=-221.0 kJ/mol
C(s)+O2(g)=CO2(g) ΠΛH=-393.5 kJ/mol
‘ρΖ¥”Π2NO(g)+2CO(g)=N2(g)+2CO2(g)ΒΡΠΛH= kJ/molΓΘ
(2)÷±Ϋ”≈≈Ζ≈ΒΣ―θΜ·ΈοΜα–Έ≥…Υα”ξΓΔΈμω≤,¥ΏΜ·ΜΙ‘≠Ζ®ΚΆ―θΜ·Έϋ ’Ζ® «≥Θ”ΟΒΡ¥ΠάμΖΫΖ®ΓΘάϊ”ΟNH3ΚΆCH4Β»ΤχΧε≥ΐ»Ξ―ΧΤχ÷–ΒΡΒΣ―θΜ·ΈοΓΘ“―÷Σ:CH4(g)+2O2(g)=CO2(g)+2H2O(l) ΠΛH1=a kJ/mol;”ϊΦΤΥψΖ¥”ΠCH4(g)+4NO(g)=CO2(g)+2H2O(l)+2N2(g)ΒΡλ ±δΠΛH2‘ρΜΙ–η“Σ≤ι―·Ρ≥Ζ¥”ΠΒΡλ ±δΠΛH3,Β±Ζ¥”Π÷–ΗςΈο÷ ΒΡΜ·―ßΦΤΝΩ ΐ÷°±»ΈΣΉνΦρ’ϊ ΐ±» ±,ΠΛH3=b kJ/mol,ΗΟΖ¥”ΠΒΡ»»Μ·―ßΖΫ≥Χ Ϋ « ,Ψί¥ΥΦΤΥψ≥ωΠΛH2= kJ/mol(”ΟΚ§aΓΔbΒΡ ΫΉ”±μ Ψ)ΓΘ
(3)œ¬±μΝ–≥ωΝΥΙΛ“Β…œΈϋ ’SO2ΒΡ»ΐ÷÷ΖΫΖ®ΓΘ
ΖΫΖ®Δώ | ”ΟΑ±Υ°ΫΪSO2ΉΣΜ·(NH4)2SO3,‘Ό―θΜ·≥…(NH4)2SO4 |
ΖΫΖ®Δρ | ”Ο…ζΈο÷ »»ΫβΤχ(÷ς“Σ≥…Ζ÷COΓΔCH4ΓΔH2)ΫΪSO2‘ΎΗΏΈ¬œ¬ΜΙ‘≠≥…ΒΞ÷ Νρ |
ΖΫΖ®Δσ | ”ΟNa2SO3»ή“ΚΈϋ ’SO2,‘ΌΨ≠ΒγΫβΉΣΜ·ΈΣH2SO4 |
ΖΫΖ®Δρ÷ς“ΣΖΔ…ζΝΥœ¬Ν–Ζ¥”Π:
2CO(g)+SO2(g)=S(g)+2CO2(g) ΠΛH=+8.0 kJ/mol
2H2(g)+SO2(g)=S(g)+2H2O(g)ΠΛH=+90.4 kJ/mol
2CO(g)+O2(g)=2CO2(g) ΠΛH=-566.0 kJ/mol
2H2(g)+O2(g)=2H2O(g) ΠΛH=-483.6 kJ/mol
‘ρS(g)”κO2(g)Ζ¥”Π…ζ≥…SO2(g)ΒΡ»»Μ·―ßΖΫ≥Χ ΫΩ…±μ ΨΈΣ ΓΘ
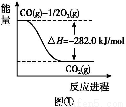
(4)Κœ≥…Α±”ΟΒΡ«βΤχΩ…“‘ΦΉΆιΈΣ‘≠Νœ÷ΤΒΟΓΘ”–ΙΊΜ·―ßΖ¥”ΠΒΡΡήΝΩ±δΜ·»γΆΦΥυ Ψ,‘ρCH4(g)”κH2O(g)Ζ¥”Π…ζ≥…CO(g)ΚΆH2(g)ΒΡ»»Μ·―ßΖΫ≥Χ ΫΈΣ ΓΘ
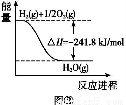
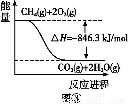
(1)ΔΌN2+O2 2NO ΔΎ-746.5
2NO ΔΎ-746.5
(2)N2(g)+O2(g)=2NO(g) ΠΛH3=b kJ/mol a-2b
(3)S(g)+O2(g)=SO2(g) ΠΛH=-574.0 kJ/mol
(4)CH4(g)+H2O(g)=CO(g)+3H2(g)ΠΛH=+161.1 kJ/mol
ΓΨΫβΈωΓΩ(1)ΔΎΑ¥ΧβΗ…Υ≥–ρΗχ3Ηω»»Μ·―ßΖΫ≥Χ Ϋ±ύΚ≈,”…Η«ΥΙΕ®¬…ΔέΓΝ2-ΔΌ-ΔΎΒΟ2NO(g)+2CO(g) N2(g)+2CO2(g)ΒΡΠΛH=[-393.5ΓΝ2-180.5-(-221.0)]kJ/mol=-746.5 kJ/molΓΘ
N2(g)+2CO2(g)ΒΡΠΛH=[-393.5ΓΝ2-180.5-(-221.0)]kJ/mol=-746.5 kJ/molΓΘ
(2)ΗυΨίΗ«ΥΙΕ®¬…ΫΪ“―÷ΣΒΡΝΫΗω»»Μ·―ßΖΫ≥Χ ΫœύΦθΩ…ΒΟ»»Μ·―ßΖΫ≥Χ ΫΈΣ2N2(g)+2O2(g)=4NO(g) ΠΛH=a kJ/mol-ΠΛH2=2b kJ/mol,‘ρΠΛH2=(a-2b)kJ/mol,Ά§ ±ΫΪ…œΟφΒΡΖΫ≥Χ ΫΦΑΠΛHΕΦ≥ΐ“‘2Ω…ΒΟ»»Μ·―ßΖΫ≥Χ ΫΓΘ
(3)ΫΪΚσΟφΒΡΝΫΗωΖΫ≥Χ ΫœύΦ”Φθ»Ξ«ΑΝΫΗωΖΫ≥Χ Ϋ‘Ό≥ΐ“‘2Ω…ΒΟœύ”ΠΒΡ»»Μ·―ßΖΫ≥Χ Ϋ:S(g)+O2(g)=SO2(g)ΠΛH=-574.0 kJ/molΓΘ
(4)Α¥ΧβΗ…Υ≥–ρΗχΆΦœώ÷–ΒΡ3Ηω»»Μ·―ßΖΫ≥Χ Ϋ±ύΚ≈,ΗυΨίΗ«ΥΙΕ®¬…,Δέ-ΔΎΓΝ3-ΔΌΒΟCH4(g)+H2O(g)=CO(g)+3H2(g)ΠΛH=[-846.3-(-241.8)ΓΝ3-(-282.0)]kJ/mol=+161.1 kJ/molΓΘ

 ΧλΧλœρ…œ“Μ±ΨΚΟΨμœΒΝ–¥πΑΗ
ΧλΧλœρ…œ“Μ±ΨΚΟΨμœΒΝ–¥πΑΗ –Γ―ß…ζ10Ζ÷÷””Π”ΟΧβœΒΝ–¥πΑΗ
–Γ―ß…ζ10Ζ÷÷””Π”ΟΧβœΒΝ–¥πΑΗ