��Ŀ����
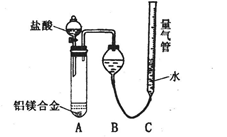
������㷺Ӧ������ҩ�ͻ�����ҵ��ijͬѧ�����üױ���������Ӧ�Ʊ������ᣬ��Ӧԭ����
ʵ�鷽����һ�����ļױ���KMnO4��Һ��100�淴Ӧһ��ʱ���ֹͣ��Ӧ�����������̷����������ͻ���δ��Ӧ�ļױ���
��֪�������������122���۵�122.4�棬��25���95��ʱ�ܽ�ȷֱ�Ϊ0.3 g��6.9 g�����������л��ﶼ�й̶��۵㡣
��1��������Ϊ ��������Ϊ ��
��2����ɫҺ��A�� �����Լ���A���Լ��� ��������
��
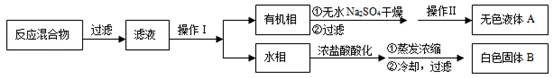
��3���ⶨ��ɫ����B���۵㣬��������115�濪ʼ�ۻ����ﵽ130��ʱ�����������ۣ���ͬѧ�Ʋ��ɫ����B�DZ�������KCl�Ļ�����������·��������ᴿ�ͼ��飬ʵ���������Ʋ���ȷ�����ڴ������ɱ������ݡ�
��4�����Ȳⶨ����ȡ1.220g��Ʒ�����100ml�״���Һ����ȡ25.00ml��Һ���ζ�������KOH�����ʵ���Ϊ2.40��10��3mol����Ʒ�б��������������ļ������ʽΪ ��������Ϊ ��������λ��Ч���֣���

ʵ�鷽����һ�����ļױ���KMnO4��Һ��100�淴Ӧһ��ʱ���ֹͣ��Ӧ�����������̷����������ͻ���δ��Ӧ�ļױ���

��֪�������������122���۵�122.4�棬��25���95��ʱ�ܽ�ȷֱ�Ϊ0.3 g��6.9 g�����������л��ﶼ�й̶��۵㡣
��1��������Ϊ ��������Ϊ ��
��2����ɫҺ��A�� �����Լ���A���Լ��� ��������
��
��3���ⶨ��ɫ����B���۵㣬��������115�濪ʼ�ۻ����ﵽ130��ʱ�����������ۣ���ͬѧ�Ʋ��ɫ����B�DZ�������KCl�Ļ�����������·��������ᴿ�ͼ��飬ʵ���������Ʋ���ȷ�����ڴ������ɱ������ݡ�
| ��� | ʵ�鷽�� | ʵ������ | ���� |
| | ����ɫ����B����ˮ�У������� �⣬ | �õ���ɫ�������ɫ��Һ | ----------------- |
| �� | ȡ������Һ���Թ��У� | ���ɰ�ɫ���� | ��Һ��Cl�� |
| �� | �����ɫ���壬 | | ��ɫ�����DZ����� |
��1����Һ������ ��2���ױ�������KMnO4��Һ����ɫ��Һ��ɫ��
��3��
��4�� [(2.40��10-3��122��4)/1.22]��100%�� 96%
��3��
| ��� | ʵ�鷽�� | ʵ������ | ���� |
| �� | ����ɫ����B����ˮ�У����ȣ��ܽ⣬ ��ȴ������ | �õ���ɫ�������ɫ��Һ | ---------- |
| �� | ȡ������Һ���Թ��У����������������ữ��AgNO3��Һ | ���ɰ�ɫ���� | ��Һ����Cl- |
| �� | �����ɫ���壬 ����ʹ���ڻ��������۵㣻 | �۵�Ϊ122.4�� | ��ɫ�����DZ����� |
��4�� [(2.40��10-3��122��4)/1.22]��100%�� 96%
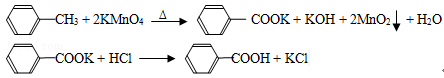
�������̴��¹���Ϊ����Ӧ��ı�����غ�δ��Ӧ�ļױ�ͨ����Һ����Ϊ�л����ˮ�࣬�л���ɷ���Ҫ�Ǽױ���ˮ����Ҫ�DZ�����أ��л���ͨ����ˮ�����Ʋ���ˮ����������İ취�������ױ������л��౽�����ͨ�������ữ��Ϊ�����ᣬͨ������Ũ��������Ũ����ӷ�����ȴ���ˣ�������ؼ�Ϊ��Ŀ��ɫ�������ʣ���ˣ�1��Ϊ��Һ������2���в����ı�����ʹ���������ɫ�����Լ���ױ�����������KMnO4��Һ���ڴ���������������KMnO4��Һ��ɫ����֤���мױ���3������KCl�Ĵ��ڼ���Cl-���Ӿͺã�����������Һ�����ݱ�����ʾ��������Ĵ��ڼ���۵㣬�л���һ���۵�ϵͣ�4����������KOH��һ��һ��Ӧ�����25ml�������������Ϊ2.40��10��3mol��100mlΪ2.40��10��3��4��mol�����ܵı���������Ϊ2.40��10��3��4��122��g���������������Ϊ[(2.40��10-3��122��4)/1.22]��100% = 96%��
�����㶨λ���л�ʵ�������̽��
�����㶨λ���л�ʵ�������̽��

��ϰ��ϵ�д�
 �Űٷֿ�ʱ����ϵ�д�
�Űٷֿ�ʱ����ϵ�д�
�����Ŀ
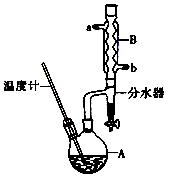
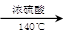 (CH3CH2CH2CH2)2O + H2O
(CH3CH2CH2CH2)2O + H2O 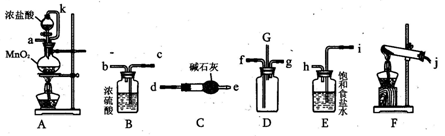
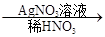 ��ɫ��������ش��������⣺
��ɫ��������ش��������⣺ ��ɫ�������йط�Ӧ�����ӷ���ʽ____________________________��
��ɫ�������йط�Ӧ�����ӷ���ʽ____________________________�� �ⶨ������������
�ⶨ������������