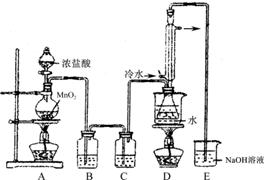
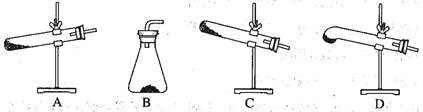
��Ŀ����
����þ�������̼�ķ�Ӧ�Ʋ⣬��Ҳ���ڶ�����̼��ȼ�գ��ҹ���������Ϊ̼���ơ������ơ�̼�е����ֻ����֡�ij��ȤС������ڶ�����̼��ȼ�պ�IJ�����ж��ԺͶ���̽����
��1���������ΪNa2CO3��Na2O��C�Ļ������ʵ�鷽������֤���е�Na2CO3��Na2O���ڴ����д��ʵ�鲽�衢Ԥ������ͽ��ۡ�����֪����ʱBaCO3������Һ��pH=9.6��
��ѡ�Լ���������ϡ���ᡢBaCl2��Һ��Ba(OH)2��Һ������pH��ֽ����ȷ��0.1�����ձ����Թܡ��ιܡ���������������ɫ��
��2���������ΪNa2CO3��Na2O��C�Ļ���Ϊ��һ���ⶨ������Na2CO3�ĺ���������������ʵ�飺
����1��ȷ��ȡw���������ܽ⡢���˺�ȷ���Ƴ�250mL��Һ��
����2��ȷ��ȡ25.00mL������Һ����ƿ�У��μӼ���ָʾ��A���μ�c mol/L�ı���������Һ��pHԼΪ8.2��̼����Ҫ������ʽΪHCO3�D����������������ΪV1mL���ٵμӼ��μ��ȣ��������������������Һ�ɻ�ɫ���ɫ��������������ΪV2mL��
����3���ظ�ʵ��3�Ρ��ζ��������
�ٲ���2�У���һ���ζ���ʹ�õ�Aָʾ��Ϊ ���ζ��յ������Ϊ ��
�ڼ���Na2CO3����������= ���ú�w��c�Ĵ���ʽ��ʾ��
��1���������ΪNa2CO3��Na2O��C�Ļ������ʵ�鷽������֤���е�Na2CO3��Na2O���ڴ����д��ʵ�鲽�衢Ԥ������ͽ��ۡ�����֪����ʱBaCO3������Һ��pH=9.6��
��ѡ�Լ���������ϡ���ᡢBaCl2��Һ��Ba(OH)2��Һ������pH��ֽ����ȷ��0.1�����ձ����Թܡ��ιܡ���������������ɫ��
| ʵ����� | Ԥ������ͽ��� |
| ����1��ȡ����������Ʒ�ڽྻ�ձ��У�������������ˮ����ֽ��裬���ã�ȡ�ϲ���Һ���á� | �в��ܵĺ�ɫ���塣 |
| ����2��ȡ��������1��Һ���Թ��У� | |
| ����3�� | |
��2���������ΪNa2CO3��Na2O��C�Ļ���Ϊ��һ���ⶨ������Na2CO3�ĺ���������������ʵ�飺
����1��ȷ��ȡw���������ܽ⡢���˺�ȷ���Ƴ�250mL��Һ��
����2��ȷ��ȡ25.00mL������Һ����ƿ�У��μӼ���ָʾ��A���μ�c mol/L�ı���������Һ��pHԼΪ8.2��̼����Ҫ������ʽΪHCO3�D����������������ΪV1mL���ٵμӼ��μ��ȣ��������������������Һ�ɻ�ɫ���ɫ��������������ΪV2mL��
����3���ظ�ʵ��3�Ρ��ζ��������
| �ζ� ���� | ������Һ �����/mL | ���ı��������� | |
| V1/mL | V2/mL | ||
| 1 | 25.00 | 15.02 | 4.97 |
| 2 | 25.00 | 14.98 | 5.03 |
| 3 | 25.00 | 13.21 | 6.75 |
�ٲ���2�У���һ���ζ���ʹ�õ�Aָʾ��Ϊ ���ζ��յ������Ϊ ��
�ڼ���Na2CO3����������= ���ú�w��c�Ĵ���ʽ��ʾ��
��1��
| ʵ����� | Ԥ����������� |
| ����2���μ�������BaCl2��Һ����������ã�ȡ�ϲ���Һ������һ֧�Թܱ��� | �а�ɫ�������ɣ�˵����������Na2CO3 |
| ����3����һƬ����PH��ֽ���ڱ������ϣ��ò�����պȡ����2�ϲ���Һ���ھ���PH��ֽ�ϣ�����ɫ�ȶ����ձ�ɫ���������ݡ� | ��Һ��PH���Դ���9.6��˵�������к���Na2O |
��2���ٷ�̪ ��dz��ɫ��Ϊ��ɫ���Ұ���Ӳ���ɫ
��5.3c/w��100%
�����������1��Na2O+H2O=2NaOH��NaOH��Na2CO3������ϡ���ᷴӦ����˲��ܵμ�ϡ���Na2CO3��BaCl2��Ba(OH)2�Ƕ��ܷ�Ӧ���ɰ�ɫ���������Ǻ�������NaOH�����Ų�����Na2O�ļ��飬��˲���2��ֻ�ܵμ�����BaCl2��Һ��������а�ɫ�������ɣ�˵�������д���Na2CO3�����ã�ȡ�ϲ���Һ������һ֧�Թܱ��ã���������ʱBaCO3������Һ��pH=9.6���粽��2���ϲ���Һ�л���NaOH������pH>9.6����˲���3��Ӧ��һƬ����pH��ֽ���ڱ������ϣ��ò�����պȡ������ϲ���Һ���ھ���pH��ֽ�ϣ�����ɫ�ȶ����ձ�ɫ���������ݣ�����ҺpH���Դ���9.6˵�������к���Na2O����2���ٲ���2�е�һ���ζ��յ��pHԼΪ8.2���պ��ڷ�̪��pH��ɫ��Χ�����ѡ���̪��ָʾ����������ļ�����Һ��dz��ɫ�����ɫ���Ұ�����ڲ���ɫ�����ǵζ��յ������ֹͣ�ζ����ڵ�3�εζ��������ݴ������Ե���Ӧ��ȥ�����ݵ�1��2�εζ������������ƽ���������V1=(15.02+14.98)mL/2=15.00mL��V2=(4.97+5.03)mL/2=5.00mL�����ڵ�1�εζ�ʱ�Ⱥ�����ӦΪNaOH+HCl=NaCl+H2O��Na2CO3+HCl=NaHCO3+NaCl����n(HCl)=n(NaOH)+n(Na2CO3)=cmol/L��15.00��10��3L��n(Na2CO3)=n(NaHCO3)����2�εζ�ʱֻ������ӦNaHCO3+HCl=NaCl+CO2��+H2O����n(HCl)=n(NaHCO3)=cmol/L��5.00��10��3L������n(Na2CO3)= n(NaHCO3)=cmol/L��5.00��10��3L��n(NaOH)= cmol/L��15.00��10��3L��cmol/L��5.00��10��3L = cmol/L��10.00��10��3L��ʽ����n(Na2CO3)=cmol/L��5.00��10��3L��250mL/25.00mL��n(NaOH)= cmol/L��10.00��10��3L��250mL/25.00mL��ʽ����m(Na2CO3)=cmol/L��5.00��10��3L��250mL/25.00mL��106g/mol��w(Na2CO3)=cmol/L��5.00��10��3L��250mL/25.00mL��106g/mol��wg��100%=5.3c/w��100%��

��ϰ��ϵ�д�
�����Ŀ
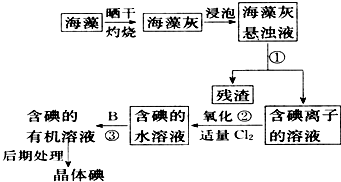
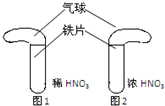
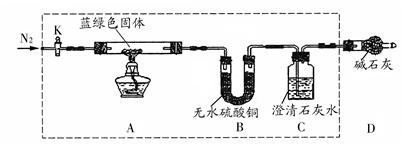
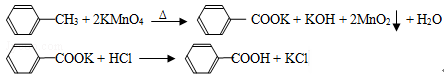
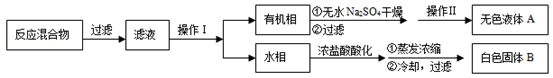

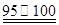 ��CCl4��S2Cl2���� 2S��Cl2
��CCl4��S2Cl2���� 2S��Cl2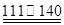 ��S2Cl2��
��S2Cl2��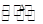 2SCl2��
2SCl2��