��Ŀ����
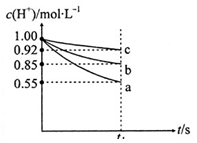
����A��B��C��D��E����������ˮ��ǿ����ʣ������������������(�������Ӳ��ظ�)��
��֪����0.1 mol/L A��Һ��pH <1���ڽ�B��Һ�ֱ�������������Һ��ϣ����а�ɫ�������ɣ�
��C��Һ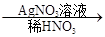 ��ɫ��������ش��������⣺
��ɫ��������ش��������⣺
��1��д���������ʵĻ�ѧʽ��A______________��B______________��
��2��д����C��Һ ��ɫ�������йط�Ӧ�����ӷ���ʽ____________________________��
��ɫ�������йط�Ӧ�����ӷ���ʽ____________________________��
��3��D��E���������б���һ����_______________��д�������������ʵ���Һ�μӵ�B��Һ�з�Ӧ�����ӷ���ʽ___________________________________________________________________��
��4���������ʵ��ȷ��C����һ��δ֪�����ʲô���ʡ�(ֻ����A��E��ѡ������Լ�)
| ������ | H+��NH4+��Mg2+��Ba2+��Al3+ |
| ������ | OH-��Cl-��HCO3-��NO3-��SO42- |
��C��Һ
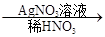 ��ɫ��������ش��������⣺
��ɫ��������ش��������⣺��1��д���������ʵĻ�ѧʽ��A______________��B______________��
��2��д����C��Һ
 ��ɫ�������йط�Ӧ�����ӷ���ʽ____________________________��
��ɫ�������йط�Ӧ�����ӷ���ʽ____________________________����3��D��E���������б���һ����_______________��д�������������ʵ���Һ�μӵ�B��Һ�з�Ӧ�����ӷ���ʽ___________________________________________________________________��
��4���������ʵ��ȷ��C����һ��δ֪�����ʲô���ʡ�(ֻ����A��E��ѡ������Լ�)
| ʵ�鲽�� | Ԥ������ͽ��� |
| ȡ����C����Һ���Թ��У� �� �� | Ԥ������ͽ���1�� �� �� Ԥ������ͽ���2�� �� �� |
(14��)(1)����H2SO4 B��Ba(OH)2 (2)Ag+��Cl����AgCl�� ����2�֣���6�֣�
(3) NH4HCO3 NH4����HCO3����Ba2����2OH����BaCO3����NH3��H2O��H2O ����2�֣���4�֣�
(4) �������м���������Ba(OH)2��Һ�� ��2�֣�
������ʼ�а�ɫ�������ɣ������������ɣ���CΪAlCl3��
������ʼ�����а�ɫ�������ɣ���CΪMgCl2 ����1�֣���2�֣�
(�������м�����ҺNH4HCO3�����������������а�ɫ�������ɣ���CΪAlCl3���������κ�������CΪMgCl2�� (����������ȷҲ����)
(3) NH4HCO3 NH4����HCO3����Ba2����2OH����BaCO3����NH3��H2O��H2O ����2�֣���4�֣�
(4) �������м���������Ba(OH)2��Һ�� ��2�֣�
������ʼ�а�ɫ�������ɣ������������ɣ���CΪAlCl3��
������ʼ�����а�ɫ�������ɣ���CΪMgCl2 ����1�֣���2�֣�
(�������м�����ҺNH4HCO3�����������������а�ɫ�������ɣ���CΪAlCl3���������κ�������CΪMgCl2�� (����������ȷҲ����)
�����������1��0.1 mol/L A��Һ��pH��1����˵��AӦ���Ƕ�Ԫǿ�ᣬ����A�����ᡣ��B��Һ�ֱ�������������Һ��ϣ����а�ɫ�������ɣ���˵��B�к���OH����Ba2������B��������������һ������������炙��Ȼ�李�
��2��C��Һ
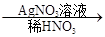 ��ɫ������˵��C�к��������ӣ���Ӧ�����ӷ���ʽ��Ag+��Cl����AgCl����
��ɫ������˵��C�к��������ӣ���Ӧ�����ӷ���ʽ��Ag+��Cl����AgCl������3������ΪHCO3����Al3�����ܴ������棬��һ������������炙��Ȼ�泥�����D��E���������б���һ����NH4HCO3�������������ʵ���Һ�μӵ�B��Һ�з�Ӧ�����ӷ���ʽNH4����HCO3����Ba2����2OH����BaCO3����NH3��H2O��H2O��
��4���������Ϸ�����֪��C���Ȼ������Ȼ�þ��������һ������������þ������������˿��Ը����������������Խ��м��飬����Ҫ����C�ijɷ֣���ȷ�IJ���Ӧ���Ǣ������м���������Ba(OH)2��Һ��������ʼ�а�ɫ�������ɣ������������ɣ���CΪAlCl3��������ʼ�����а�ɫ�������ɣ���CΪMgCl2��
(�������м�����ҺNH4HCO3�����������������а�ɫ�������ɣ���CΪAlCl3���������κ�������CΪMgCl2��)
�����������ۺ���ǿ���ѶȽϴ�ѧ����˼ά����Ҫ��ߣ�ѧ�����÷֡�������ע�ض�ѧ������֪ʶ���̺ͼ����ͬʱ�����ض�ѧ�����������������ͽ��ⷽ����ָ����ѵ��������������ѧ���������������淶�Ͻ���ʵ���������������������ѧ����ѧ�����������ѧ����ѧϰЧ�ʺ�ѧϰ�����ԡ�ע�����֪ʶ�Ļ��ۡ��ܽ�����á�

��ϰ��ϵ�д�
�����Ŀ