��Ŀ����
��һ������þ��ͭ��ɵĻ������뵽ϡ�����У�������ȫ�ܽ⣨���跴Ӧ�л�ԭ����ֻ��NO������Ӧ�����Һ�м���3 mol��L NaOH��Һ��������ȫ��������ɳ�����������ԭ�Ͻ����������5��1 g��������������ȷ����
| A�������ɵij������ﵽ���ʱ������NaOH��Һ�����V��100 mL |
| B��������ȫ���ܽ�ʱ�ռ���NO��������һ��Ϊ2��24 L |
| C���μӷ�Ӧ�Ľ�����������3��6 g<m<9��6 g |
| D��������ȫ���ܽ�ʱ���μӷ�Ӧ����������ʵ���һ����0��4 mol |
B
����������������֪�����ɳ�����������ԭ�Ͻ����������5.1g����Ϊ���ӵ����������ӵ��������������������ӵ����ʵ���Ϊ0.3mol��Ҳ�Ƿ�Ӧ��ת�Ƶ��ӵ����ʵ�����A�����������������ӵ����ʵ���Ϊ0.3mol������Ҫ�������Ƶ������100mL�����Ե����ɵij������ﵽ���ʱ������NaOH��Һ�����V��100 mL����ȷ��B����Ӧ��ת�Ƶ��ӵ����ʵ�����0.3mol����NO�����ʵ�����0.1mol����δָ����״������������������һ����2.24L������C��������ȫ����þ����������Ϊ0.3mol/2��24g/mol=3.6g,������ȫ����ͭ����������Ϊ0.3mol/2��64g/mol=9.6g�����Թ������������3.6g��9.6g֮�䣬��ȷ��D������ȫ���ܽ�ʱ����ԭ������Ҳ�����ɵ�NO�����ʵ�����0.1mol��δ����ԭ�����ἴ���������Ʒ�Ӧ���������Ƶ����ʵ�����0.3mol�����Թ���������0.4mol����ȷ����ѡB��

��ϰ��ϵ�д�
 �����������һ��һ��ϵ�д�
�����������һ��һ��ϵ�д�
�����Ŀ

 Cu2O��H2������ʯīӦ���Դ��________��������ͭ�缫�ϵĵ缫��ӦʽΪ________���������У���������Χ��ҺpH________(��������С�����䡱)��
Cu2O��H2������ʯīӦ���Դ��________��������ͭ�缫�ϵĵ缫��ӦʽΪ________���������У���������Χ��ҺpH________(��������С�����䡱)��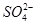 )��������NaOH��������ɫ���������ˣ�ϴ�ӣ����գ��õ�CuO 12��0 g������������ΪNO��NO2�Ļ����������Ϊ1��1����V����Ϊ
)��������NaOH��������ɫ���������ˣ�ϴ�ӣ����գ��õ�CuO 12��0 g������������ΪNO��NO2�Ļ����������Ϊ1��1����V����Ϊ (NxOy)�� H2O(��ƽʱx��y�þ�����ֵ��ʾ����������
(NxOy)�� H2O(��ƽʱx��y�þ�����ֵ��ʾ����������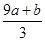 �� mol
�� mol