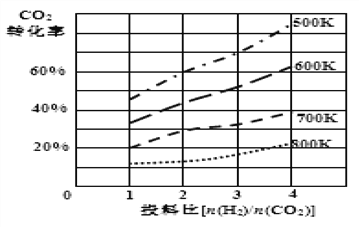
��Ŀ����
����Ŀ��ʵ����Ҫ����100 mL 2 mol/L NaCl��Һ����ش��������⣺
��1�����ƹ�������Ҫʹ�õ���Ҫ�������������ձ�������������ͷ�ιܡ���Ͳ��________��
��2����������ƽ��ȡ�Ȼ��ƹ��壬������Ϊ__________ g��
��3��������Ҫ�����������ȷ˳����___________������ţ���
�ٳ�ȡһ���������Ȼ��ƣ������ձ��У�����������ˮ�ܽ�
�ڼ�ˮ��Һ��������ƿ���̶�����1~2����ʱ�����ý�ͷ�ιܵμ�����ˮ����Һ����̶�������
�۽���Һת�Ƶ�����ƿ��
�ܸǺ�ƿ�����������µߵ���ҡ��
������������ˮϴ���ձ��ڱںͲ�����2~3�Σ�ϴ��Һת�Ƶ�����ƿ��
��4�����ʵ�������ȱ�ٲ���ݣ������������Һ�����ʵ���Ũ��_______���ƫ�ߡ���ƫ�͡�����Ӱ�족����ͬ����������ʱ��������ƿ�̶��ߣ������������Һ�����ʵ���Ũ��_________��
���𰸡�100mL����ƿ 11.7�٢ۢݢڢ�ƫ��ƫ��
��������
(1)��������һ�����ʵ���Ũ�ȵ���Һ�IJ���ѡ��ʹ�õIJ���������
(2)����100 mL 2 mol/L NaCl��Һ�к��������Ȼ��Ƶ����ʵ���������Ȼ��Ƶ�������
(3)��������һ�����ʵ���Ũ�ȵ���Һ�IJ���Բ�������������
(4)����c=![]() ��������ʱ���ؼ�Ҫ�����ƹ���������n��V�����ı仯����n������ֵС��V������ֵ��ʱ������ʹ������ҺŨ��ƫС����n������ֵ���V������ֵСʱ������ʹ������ҺŨ��ƫ����
��������ʱ���ؼ�Ҫ�����ƹ���������n��V�����ı仯����n������ֵС��V������ֵ��ʱ������ʹ������ҺŨ��ƫС����n������ֵ���V������ֵСʱ������ʹ������ҺŨ��ƫ����
(1) ����100 mL 2 mol/L ��NaCl��Һ�����������м��㡢��ȡ��ϡ�͡���Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ���������Ҫ����������ƽ��ҩ�ס�����������ͷ�ιܡ��ձ���100 mL����ƿ������Ҫ�IJ���������������������ͷ�ιܡ��ձ���100 mL����ƿ��
��ˣ�������ȷ������100mL����ƿ��
(2) ����100 mL 2 mol/L NaCl��Һ����Ҫ�Ȼ��Ƶ�����Ϊ��0.1L![]() 58.5g/mol=11.7g��
58.5g/mol=11.7g��
��ˣ�������ȷ������11.7��
(3)����һ�����ʵ���Ũ�ȵ���Һ�IJ���Ϊ:���㡢��ȡ��ϡ�͡���Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ��������Բ���˳��Ϊ���٢ۢݢڢܣ�
��ˣ�������ȷ�������٢ۢݢڢ���
(4)û��ϴ���ձ��Ͳ���������������ƿ���Ȼ��Ƶ�����ƫС��������Һ��Ũ��ƫ��������ʱ�����ӿ̶�����Һ���ڿ̶����·�����Һ�����ƫС��������Һ��Ũ��ƫ����
��ˣ�������ȷ������ƫ����ƫ����

 ���Ž�������С״Ԫϵ�д�
���Ž�������С״Ԫϵ�д�����Ŀ����ʹ������к͵ζ����ⶨ���۰״�������![]()
��![]() ʵ�鲽��
ʵ�鲽��
��1������100mL����״���Һ���� ______ ![]() ����������
����������![]() ��ȡ
��ȡ![]() ���۰״ף����ձ�����ˮϡ�ͺ�ת�Ƶ� ______
���۰״ף����ձ�����ˮϡ�ͺ�ת�Ƶ� ______ ![]() ����������
����������![]() �ж��ݣ�ҡ�ȼ��ô���״���Һ��
�ж��ݣ�ҡ�ȼ��ô���״���Һ��
��2������ʽ�ζ���ȡ����״���Һ![]() ����ƿ�У������еμ�2�� _____ ��ָʾ����
����ƿ�У������еμ�2�� _____ ��ָʾ����
��3����ȡʢװ![]() NaOH��Һ�ļ�ʽ�ζ��ܵij�ʼ����
NaOH��Һ�ļ�ʽ�ζ��ܵij�ʼ����![]() ���Һ��λ����ͼ��
���Һ��λ����ͼ��![]() ʾ�����ʱ�Ķ���Ϊ ______ mL��
ʾ�����ʱ�Ķ���Ϊ ______ mL��
��4���ζ�![]() �� ______ ʱ��ֹͣ�ζ�������¼NaOH��Һ���ն���
�� ______ ʱ��ֹͣ�ζ�������¼NaOH��Һ���ն���![]() �ظ��ζ�3�Σ�
�ظ��ζ�3�Σ�
��5��ʵ���¼
�ζ�����ʵ������ | 1 | 2 | 3 | 4 |
|
|
|
|
|
|
|
|
|
|
��![]() ���ݴ���������
���ݴ���������
��ʵ���������ݣ��ɵ�![]() ���۰״�
���۰״�![]() ______
______ ![]() �����۰״�������
�����۰״�������![]() ______
______ ![]() ��
��
��6���ڱ�ʵ��ĵζ������У����в�����ʹʵ����ƫ����� ______ (��д���![]() ��
��
![]() ��ʽ�ζ����ڵζ�ʱδ�ñ�NaOH��Һ��ϴ
��ʽ�ζ����ڵζ�ʱδ�ñ�NaOH��Һ��ϴ
![]() ��ʽ�ζ��ܵļ����ڵζ�ǰ�����ݣ��ζ���������ʧ
��ʽ�ζ��ܵļ����ڵζ�ǰ�����ݣ��ζ���������ʧ
![]() ��ƿ�м������״���Һ���ټ�����ˮ
��ƿ�м������״���Һ���ټ�����ˮ
![]() ��ƿ�ڵζ�ʱ����ҡ����������Һ�彦����
��ƿ�ڵζ�ʱ����ҡ����������Һ�彦����