��Ŀ����
����Ŀ���о�CO2�����öԴٽ���̼���Ĺ���������Ҫ�����塣
��1����֪��1 mol H2��1 mol O2��Һ̬ˮ��1 mol O��H��ʹ֮��Ϊ��̬ԭ������������ֱ�Ϊ436 kJ��496 kJ��462 kJ��CH3OH(g)��ȼ����Ϊ627 kJ��mol��1����CO2(g)��3H2(g)��CH3OH(g)��H2O(l) H��___________kJ��mol��1
��2����ȼú�����е�CO2ת��Ϊ�����ѵķ�Ӧԭ��Ϊ��2CO2(g)�� 6H2(g) ![]() CH3OCH3(g)��3H2O(l)
CH3OCH3(g)��3H2O(l)
�� �÷�Ӧƽ�ⳣ������ʽK��_______________��
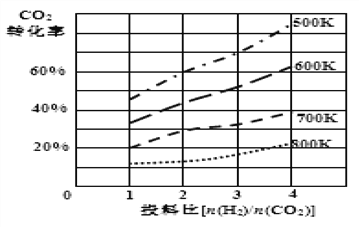
�� ��֪��ijѹǿ�£��÷�Ӧ�ڲ�ͬ�¶ȡ���ͬͶ�ϱ�ʱ��CO2��ת������ͼ��ʾ���÷�Ӧ��H________0 (����������������)��
���¶Ȳ��䣬��С��ӦͶ�ϱ�[n(H2)/n(CO2)]����K��_____(����������������С������������)��
��ij�¶��£������һ�����ܱ�������ͨ��CO2(g)��H2(g)����������Ӧ�����������������ٷ����仯ʱ����֤���������淴Ӧ�ﵽƽ�������__________��
A��������̼��Ũ�� B�������е�ѹǿ
C��������ܶ� D��CH3OCH3��H2O�����ʵ���֮��
��3���Լ��ѡ�����������������ҺΪԭ�ϵ�ȼ�ϵ��Ϊ��Դ����ʯīΪ�缫���500mL���з�̪��NaCl��Һ��װ����ͼ��ʾ��
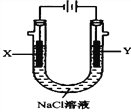
��д����������Y�缫�����۲쵽������______________����ȼ�ϵ������2.8L O2(��״����)ʱ�������ʱNaCl��Һ��pH=________(������Һ��������䣬����ȫ������Һ���ݳ�)��
���𰸡� ��93 c(CH3OCH3)/ [c2(CO2)��c6(H2)] �� ���� ABC Y�缫������Һ��������������ϲ��ֳʻ���ɫ 14
��������(1)��֪��1 mol H2��1 mol O2��Һ̬ˮ��1 mol O��H��ʹ֮��Ϊ��̬ԭ������������ֱ�Ϊ436 kJ��496 kJ��462 kJ�����˿�д��H2ȼ������Һ̬ˮ���Ȼ�ѧ����ʽΪ��2H2(g)��O2(g)= 2H2O(l) ��H=��480kJ��mol��1����CH3OH(g)��ȼ����Ϊ627 kJ��mol��1���ɵ�CH3OH(g)ȼ�յ��Ȼ�ѧ����ʽΪ��CH3OH(g)��3/2O2(g)=CO2(g)��2H2O(l) ��H=��627kJ��mol��1���ɸ�˹���ɿɽ��Т١�3/2���ڣ�����CO2(g)��3H2(g)��CH3OH(g)��H2O(l) H����93 kJ��mol��1��
(2)�ٸ���ƽ�ⳣ���ı���ʽ����ɵ�����Ӧ2CO2(g)�� 6H2(g) ![]() CH3OCH3(g)��3H2O(l)��ƽ�ⳣ������ʽK��c(CH3OCH3)/ [c2(CO2)��c6(H2)]���ڸ���ͼ��������¶�Խ�ߣ�CO2��ת����Խ�ͣ�˵�������¶ȣ�ƽ�������ƶ�����÷�ӦΪ���ȷ�Ӧ����H�� 0�����¶Ȳ��䣬��С��ӦͶ�ϱ�[n(H2)/n(CO2)]��CO2��ת���ʼ�С��˵��ƽ�������ƶ�����ƽ�ⳣ��K�Dz���ģ���Ϊƽ�ⳣ��Kֻ���¶ȵĸı���仯���۸��ݻ�ѧƽ��״̬���������������£�A����c(CO2)����ʱ��˵����Ӧ�Ѵ�ƽ�⣬��A��ȷ��B�������һ�����ܱ������У��÷�Ӧǰ�������������ȣ����Ե�ѹǿ����ʱ��˵����Ӧ�Ѵ�ƽ�⣬��B��ȷ��C�����ݦ�=m/V�������һ�����ܱ������У�V���䣬��������ˮ��Һ�壬���������������m��仯�������ܶȦѵĸı䣬�ʵ��ܶȦѲ���ʱ��˵����Ӧ�Ѵ�ƽ�⣬��C��ȷ��D�����Ǵӷ�Ӧ��CO2(g)��H2(g)��ʼ���еķ�Ӧ������������CH3OCH3��H2O�����ʵ���֮��ʼ�ն���1:3���Dz���ģ���CH3OCH3��H2O�����ʵ���֮�Ȳ���ʱ������˵����Ӧ�Ѵ�ƽ�⣬��D������ȷ��ΪABC��
CH3OCH3(g)��3H2O(l)��ƽ�ⳣ������ʽK��c(CH3OCH3)/ [c2(CO2)��c6(H2)]���ڸ���ͼ��������¶�Խ�ߣ�CO2��ת����Խ�ͣ�˵�������¶ȣ�ƽ�������ƶ�����÷�ӦΪ���ȷ�Ӧ����H�� 0�����¶Ȳ��䣬��С��ӦͶ�ϱ�[n(H2)/n(CO2)]��CO2��ת���ʼ�С��˵��ƽ�������ƶ�����ƽ�ⳣ��K�Dz���ģ���Ϊƽ�ⳣ��Kֻ���¶ȵĸı���仯���۸��ݻ�ѧƽ��״̬���������������£�A����c(CO2)����ʱ��˵����Ӧ�Ѵ�ƽ�⣬��A��ȷ��B�������һ�����ܱ������У��÷�Ӧǰ�������������ȣ����Ե�ѹǿ����ʱ��˵����Ӧ�Ѵ�ƽ�⣬��B��ȷ��C�����ݦ�=m/V�������һ�����ܱ������У�V���䣬��������ˮ��Һ�壬���������������m��仯�������ܶȦѵĸı䣬�ʵ��ܶȦѲ���ʱ��˵����Ӧ�Ѵ�ƽ�⣬��C��ȷ��D�����Ǵӷ�Ӧ��CO2(g)��H2(g)��ʼ���еķ�Ӧ������������CH3OCH3��H2O�����ʵ���֮��ʼ�ն���1:3���Dz���ģ���CH3OCH3��H2O�����ʵ���֮�Ȳ���ʱ������˵����Ӧ�Ѵ�ƽ�⣬��D������ȷ��ΪABC��
(3)��װ��ͼ��֪��Y�缫���Դ������������Ϊ���������NaCl��Һ��������ӦʽΪ2Cl����2e��=Cl2�������Կɹ۲쵽������Ϊ�缫�������ݲ������ϲ�����ʻ���ɫ����ȼ�ϵ������2.8L O2(��״����)ʱ����·��ת�Ƶĵ���n(e- )=![]() =0.5mol,���ݵ���ת���غ㣬��ϵ��NaCl��Һ�ķ�Ӧ����ʽ2NaCl��2H2O
=0.5mol,���ݵ���ת���غ㣬��ϵ��NaCl��Һ�ķ�Ӧ����ʽ2NaCl��2H2O![]() 2NaOH��H2����Cl2��,�ɵô�ʱ��Һ������n(OH-)=0.5mol��c(OH-)=0.5mol/0.5L=1.0mol/L��������Һ��pH=14��
2NaOH��H2����Cl2��,�ɵô�ʱ��Һ������n(OH-)=0.5mol��c(OH-)=0.5mol/0.5L=1.0mol/L��������Һ��pH=14��