��Ŀ����
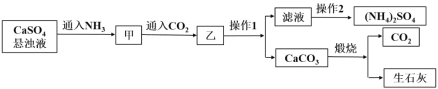
����Ŀ����һ�ݳ�����Һ�����ܺ��� Na����K����NH4+��Ca2����Fe3����SO42����CO32����SO32����Cl����I���е������֣������ӵ����ʵ���Ũ�Ⱦ�Ϊ 0.1mol��L��1(������ˮ���ˮ�ĵ���)��������Һ�м�������������ữ BaCl2��Һ���������ɡ���ȡ����ԭ��Һ����Ʋ��������ʵ�飺
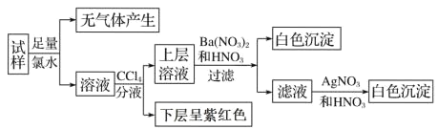
�����ԭ��Һ���ж��в���ȷ����
A.�Ƿ���� Na����K����Ҫͨ����ɫ��Ӧ��ȷ��
B.ͨ�� CCl4�����ɫ�仯�����жϳ���Һ�� I���϶�����
C.������������ˮ�����������˵����Һ�� CO32���϶�������
D.�϶������ڵ������� Ca2����Fe3����SO42����CO32����Cl��
���𰸡�A
��������
������Һ��������Һ�м��������BaCl2������Ļ����Һ���ް�ɫ�������ɣ���SO42-����������ˮ�������壬����CO32-����Һ�����Ȼ�̼��Һ���²��Ϻ�ɫ������I-���ϲ�����ᱵ��ϡ�����а�ɫ��������SO32-����Ca2+��Fe3+����Һ��һ���������ӣ�������Ũ�ȶ�Ϊ0.1molL-1�����ݵ���غ㣬һ������NH4+��Na+��K+��һ��������Cl-����Һ�м������ữ���������а�ɫ�������ǹ����м���ˮʱ��������룬�����ҺΪ�����������
A. ������Һ������Ũ�Ⱦ�Ϊ0.1mol/L������Һ�ʵ����ԣ���Һ��һ������ Na����K��������Ҫ��ɫ��Ӧ��ȷ����A�����
B. ͨ��CCl4 �����ɫ�仯�����жϳ���Һ�� I���϶����ڣ�B����ȷ��
C. ������������Cl2ˮ��û�����������˵����CO32����C����ȷ��
D. �ɷ�����֪���϶������ڵ�������Ca2����Fe3����SO42����CO32����Cl����D����ȷ��
��ѡA��

����Ŀ����X��Y��Z��W���ֶ���������Ԫ�أ�ԭ��������������Ԫ��������ԭ�ӣ���������ṹ���±���ʾ��
Ԫ�ر�� | Ԫ��������ԭ�ӣ���������ṹ |
X | ԭ�Ӻ���û������ |
Y | �����µ���Ϊ˫ԭ�ӷ��ӣ������к���3�Թ��õ��Ӷ� |
Z | ���ڲ��������������2�� |
W | ������Ԫ����ԭ�Ӱ뾶��С |
��ش�
��1��д��Ԫ��Z�����ڱ��е�λ��______������Ԫ��W��ԭ�ӽṹʾ��ͼ______��
��2��Y��W��ȣ���̬�⻯���ȶ��Խ�������________���ѧʽ����ͬ������Ԫ������������Ӧˮ�������Ը�ǿ����__________��
��3��X��Y��Z����Ԫ�ؿ����γɻ�����ZYX2�����뻯����XW������Ӧ�����������Σ�д���÷�Ӧ�Ļ�ѧ����ʽ________________��