��Ŀ����
����Ŀ�����ֶ�����Ԫ��X��Y��Z��W��Q��ԭ��������������X��Y�Ƿǽ���Ԫ��X��Y��QԪ�ص�ԭ������ܼ��ϵ�������ȣ�ZԪ��ԭ�ӵ������������Ǵ�����������WԪ��ԭ�Ӻ��������ֲ�ͬ���ܼ���ԭ����p�Dz���s�Dz����������ȣ�QԪ�ص����ֱܷ���I1=496��I2=4562��I3=6912���ش��������⣺
(1)��̬Qԭ�ӵĺ�������Ų�ʽ��____________________��
(2)Q��W�γɵĻ�����Q2W2�еĻ�ѧ��������______________��
(3)Y�����Ԫ���γ�YF3���÷��ӵĿռ乹����_______���÷�������______����(����ԡ��Ǽ��ԡ�)��Y��X���γɾ�������ṹ�Ļ�����Y2X6���ýṹ��Y����______�ӻ���
(4)Y(OH)3��һԪ���ᣬ����Yԭ����ȱ���Ӷ����γ���λ����д��Y(OH)3��ˮ��Һ�еĵ��뷽��ʽ_______________��
(5) Z��һ�ֵ��ʾ����ṹ����ͼ��ʾ��
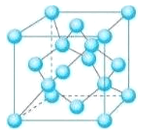
�ٸõ��ʵľ�������Ϊ___________��
�ں�1 mol Zԭ�ӵĸþ����й���_____mol��ѧ����
�ۼ�֪Z�����ԭ������ΪM��ԭ�Ӱ뾶Ϊr pm������٤��������ֵΪNA����þ�����ܶ�Ϊ____g��cm-3��
���𰸡� [Ne]3s1(��1s22s22p63s1) ���Ӽ����Ǽ��Ծ��ۼ� ƽ�������� �Ǽ��� ��sp3 B(OH)3+H2O![]() [B(OH)4]-+H+ ԭ�Ӿ��� 2
[B(OH)4]-+H+ ԭ�Ӿ��� 2 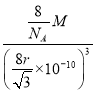 ��
��![]() ��1030
��1030
��������ZԪ��ԭ�ӵ������������Ǵ�������������ZΪ̼Ԫ�أ�WԪ��ԭ�Ӻ��������ֲ�ͬ���ܼ���ԭ����p�Dz���s�Dz����������ȣ���������Ų�ʽΪ1s22s22p4��ΪOԪ�أ�QԪ�ص����ֱܷ���I1=496��I2=4562��I3=6912��˵���������1�����ӣ���QΪNaԪ�أ��ٽ��X��Y��Z��W��Q��ԭ��������������X��Y�Ƿǽ���Ԫ��X��Y��QԪ�ص�ԭ������ܼ��ϵ�������ȿ�֪XΪH��YΪB��
(1)Na�ĺ˵����Ϊ11����̬Naԭ�ӵĺ�������Ų�ʽ��[Ne]3s1(��1s22s22p63s1) ��
(2) Na��O�γɵĻ�����Na2O2�к���Na+��O22-����������ѧ�����������Ӽ��ͷǼ��Թ��ۼ���
(3)BF3��������Bԭ�ӹµ��Ӷ���=![]() =0���۲���Ӷ���=3+0=3����Bԭ�Ӳ�ȡsp2�ӻ���VSEPR����Ϊƽ�������Σ����ӵ����幹��Ϊƽ�������Σ�BF3��BԪ�ػ��ϼ�Ϊ+3��Bԭ�������3������ȫ���ɼ���Ϊ�Ǽ��Է��ӣ���B2H6��������Bԭ�ӹµ��Ӷ���=
=0���۲���Ӷ���=3+0=3����Bԭ�Ӳ�ȡsp2�ӻ���VSEPR����Ϊƽ�������Σ����ӵ����幹��Ϊƽ�������Σ�BF3��BԪ�ػ��ϼ�Ϊ+3��Bԭ�������3������ȫ���ɼ���Ϊ�Ǽ��Է��ӣ���B2H6��������Bԭ�ӹµ��Ӷ���=![]() =0���۲���Ӷ���=4+0=4����Bԭ�Ӳ�ȡsp3�ӻ���
=0���۲���Ӷ���=4+0=4����Bԭ�Ӳ�ȡsp3�ӻ���
(4)B(OH)3��Bԭ����ȱ���Ӷ�����ˮ�������OH-����ԭ�Ӽ��γ���λ�����ٽ�ˮ�ĵ��룬ʹ����Һ�����ԣ���B(OH)3��ˮ��Һ�еĵ��뷽��ʽΪB(OH)3+H2O![]() [B(OH)4]-+H+ ��
[B(OH)4]-+H+ ��
(5) ��̼ԭ�Ӽ�ͨ��C��C���γ�������״�ṹ���˾�������Ϊԭ�Ӿ��壻
���ɾ����ṹ��֪��ÿ��̼ԭ��ƽ���γ�2��C��C������1mol Cԭ�ӵĸþ����й���2mol��ѧ����
��������̼ԭ����ĿΪ4+8��![]() +6��
+6��![]() =8����������Ϊ8��
=8����������Ϊ8��![]() g��������Խ����ϵ�Cԭ�����ڣ���Cԭ�Ӱ뾶Ϊ(r��10-10)cm������Խ��߳���Ϊ8(r��10-10)cm�����ⳤΪ
g��������Խ����ϵ�Cԭ�����ڣ���Cԭ�Ӱ뾶Ϊ(r��10-10)cm������Խ��߳���Ϊ8(r��10-10)cm�����ⳤΪ cm����þ�����ܶ�Ϊ[8��
cm����þ�����ܶ�Ϊ[8��![]() g]��[
g]��[ ]3cm3=
]3cm3=![]() ��1030g��cm-3��
��1030g��cm-3��

����Ŀ����ͼ��a��b�ֱ���ԭ��ص���������ͨ��·����a�����������ӣ�b�����ܽ⣬������һ�������( )
a���� | b���� | a�缫 | Z��Һ | |
A | п | ʯī | ���� | CuSO4 |
B | ʯī | ʯī | ���� | NaOH |
C | �� | �� | ���� | AgNO3 |
D | ͭ | ʯī | ���� | CuCl2 |

A. A B. B C. C D. D
����Ŀ����֪������Ϣ����1 mol N2�Ĺ��ۼ���������946 kJ��������1 mol H2�Ĺ��ۼ���������436 kJ���������γ�1 mol NH3�еĻ�ѧ���ͷ�1 173 kJ���������ڽ�һ������N2��H2Ͷ��һ�ܱ������У���һ�������½��з�Ӧ������й��������£�
N2(mol��L��1) | H2(mol��L��1) | NH3(mol��L��1) | |
��ʼʱ | 3 | 3 | 0 |
2sĩ | 2.6 | 1.8 | 0.8 |
��������������ݻش����⣺
(1)��H2��ʾ�÷�Ӧ2 s�ڵ�ƽ����Ӧ����Ϊ________
(2)______(��ܡ����ܡ�)ȷ�ϸ÷�Ӧ2 sĩ�Ѵﻯѧƽ��״̬��
(3)д���÷�Ӧ�Ļ�ѧ����ʽ��
________________________________________________________________________��
(4)�������������ɰ����Ĺ���______(��ͷš������ա�)������
����Ŀ����.�ߴ���������þ�㷺Ӧ����ҽҩ������������þ�����ο�ʯ����������������þ����Ҫ����������ͼ��ʾ��
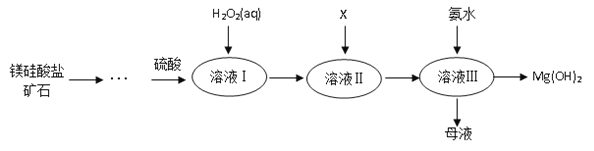
��֪��
����Һ���г���Mg2+��SO42�⣬����������Fe3+��Al3+��Fe2+�����ӣ�
�ڳ����£����ֽ������ӿ�ʼ�����ͳ�����ȫʱ��pH���±���ʾ��
�������� | Fe3+ | Al3+ | Fe2+ | Mg2+ |
��ʼ����ʱ��pH | 1.5 | 3.3 | 6.5 | 9.4 |
������ȫʱ��pH | 3.7 | 5.2 | 9.7 | 12.4 |
��ش��������⣺
��1��þ��Ԫ�����ڱ��е�λ��_______________��
��2������Һ���м����Լ�X��_____________��������_________________________��
��3�������в���H2O2����ĺ����___________________________________��
��4��˵��ĸҺ��һ����;___________________��
��5������H2O2��Һ������Ӧ�����ӷ���ʽ��___________________��
��.��ˮ�к��зḻ��þ��Դ��ijͬѧ����˴�ģ�⺣ˮ���Ʊ�MgO��ʵ�鷽����
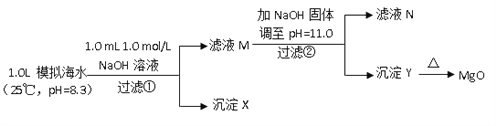
ģ�⺣ˮ�е�����Ũ��/ ��mol/L�� | Na+ | Mg2+ | Ca2+ | Cl�� | HCO3�� |
0.439 | 0.050 | 0.011 | 0.560 | 0.001 |
ע����Һ��ij�����ӵ�Ũ��С��1.0��10��5mol/L������Ϊ�����Ӳ����ڣ�ʵ������У�������Һ������䡣
��֪��Ksp(CaCO3)��4.96��10��9��Ksp(MgCO3)��6.82��10��6��Ksp [Ca(OH)2]��4.68��10��6��Ksp [Mg(OH)2]��5.61��10��12����ش��������⣺
��6��������XΪ_____________��
��7����ҺN��Ca2��Ũ��Ϊ_______________��
��8����NaOH�������pH=12.5�Ƿ����______����ǡ�������ԭ����________��
����Ŀ�����屽��ȩ��![]() ���������ϡ�Ⱦ�ϡ��л��ϳ��м��壬���±Ƚ��ȶ��������ױ�������������ʵķе����£�101kPa����
���������ϡ�Ⱦ�ϡ��л��ϳ��м��壬���±Ƚ��ȶ��������ױ�������������ʵķе����£�101kPa����
���� | �е�/�� | ���� | �е�/�� |
�� | 58.8 | 1��2���������� | 83.5 |
����ȩ | 179 | ���屽��ȩ | 229 |
��ʵ�����Ʊ��������£�
����1��������ƿ�е�һ����ȵ���ˮAlCl3��1��2����������ͱ���ȩ��ֻ�Ϻ�������60�棬�����μӾ�Ũ����������Һ�壬���·�Ӧһ��ʱ�䣬��ȴ��
����2������Ӧ����ﻺ������һ������ϡ�����У����衢���á���Һ���л�����10%NaHCO3��Һϴ�ӡ�
����3����ϴ�ӵ��л������������ˮMgSO4���壬����һ��ʱ�����ˡ�
����4����ѹ�����л��࣬�ռ���Ӧ��֡���ʵ��װ�ü���ͼ��

��1������A������Ϊ___________________��1��2����������ĵ���ʽΪ__________��
��2��ʵ��װ���������ܵ���Ҫ������________����ˮ��Ϊ____���a����b������
��3������1��Ӧ����ʽΪ____________________��Ϊ����β����ƿ�е���ҺӦΪ________����Ӧ�����ӷ���ʽΪ________________��
��4��ˮԡ���ȵ��ŵ���__________________________��
��5������2����10%NaHCO3��Һϴ���л��࣬��Ϊ�˳�ȥ�����л����______(�ѧʽ)��
��6������4�в��ü�ѹ����������Ϊ�˷�ֹ_______________��

