��Ŀ����
����Ŀ�����屽��ȩ��![]() ���������ϡ�Ⱦ�ϡ��л��ϳ��м��壬���±Ƚ��ȶ��������ױ�������������ʵķе����£�101kPa����
���������ϡ�Ⱦ�ϡ��л��ϳ��м��壬���±Ƚ��ȶ��������ױ�������������ʵķе����£�101kPa����
���� | �е�/�� | ���� | �е�/�� |
�� | 58.8 | 1��2���������� | 83.5 |
����ȩ | 179 | ���屽��ȩ | 229 |
��ʵ�����Ʊ��������£�
����1��������ƿ�е�һ����ȵ���ˮAlCl3��1��2����������ͱ���ȩ��ֻ�Ϻ�������60�棬�����μӾ�Ũ����������Һ�壬���·�Ӧһ��ʱ�䣬��ȴ��
����2������Ӧ����ﻺ������һ������ϡ�����У����衢���á���Һ���л�����10%NaHCO3��Һϴ�ӡ�
����3����ϴ�ӵ��л������������ˮMgSO4���壬����һ��ʱ�����ˡ�
����4����ѹ�����л��࣬�ռ���Ӧ��֡���ʵ��װ�ü���ͼ��

��1������A������Ϊ___________________��1��2����������ĵ���ʽΪ__________��
��2��ʵ��װ���������ܵ���Ҫ������________����ˮ��Ϊ____���a����b������
��3������1��Ӧ����ʽΪ____________________��Ϊ����β����ƿ�е���ҺӦΪ________����Ӧ�����ӷ���ʽΪ________________��
��4��ˮԡ���ȵ��ŵ���__________________________��
��5������2����10%NaHCO3��Һϴ���л��࣬��Ϊ�˳�ȥ�����л����______(�ѧʽ)��
��6������4�в��ü�ѹ����������Ϊ�˷�ֹ_______________��
���𰸡� ��Һ©�� ![]() ���������������� b
���������������� b 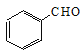 +Br2
+Br2 ![]()
![]() +HBr������ʽ��������дAlCl3��1�֣� NaOH H++OH��=H2O��Br2+2OH��=Br��+BrO��+H2O (��д�ɲ�д) ���Ⱦ��ȣ��¶ȱ��ڿ��� Br2 ���屽��ȩ������
+HBr������ʽ��������дAlCl3��1�֣� NaOH H++OH��=H2O��Br2+2OH��=Br��+BrO��+H2O (��д�ɲ�д) ���Ⱦ��ȣ��¶ȱ��ڿ��� Br2 ���屽��ȩ������
����������1��װ��������A������Ϊ��Һ©����1��2����������ĵ���ʽΪ![]() ��
��
��2�������ӷ���Ϊʹ���ַ�Ӧ��Ӧ����������������������ʣ�����ˮ�ķ����ǵͽ��߳������ˮ��Ϊb��
��3������1��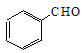 �ڴ���AlCl3�������뷢��ȡ����Ӧ����
�ڴ���AlCl3�������뷢��ȡ����Ӧ����![]() ��HBr����Ӧ����ʽΪ
��HBr����Ӧ����ʽΪ +Br2
+Br2 ![]()
![]() +HBr��������NaOHΪ���պ���HBr��Br2��β�����漰��Ӧ�����ӷ���ʽΪH++OH��=H2O��Br2+2OH��=Br��+BrO��+H2O��
+HBr��������NaOHΪ���պ���HBr��Br2��β�����漰��Ӧ�����ӷ���ʽΪH++OH��=H2O��Br2+2OH��=Br��+BrO��+H2O��
��4������ˮԡ���ȵ��ŵ������Ⱦ��ȣ��¶ȱ��ڿ��ƣ�
��5����Ӧ����ﺬ���壬��������һ������̼�����ƣ�����Br2��Ӧ��ȥ��
��6����ѹ���ɽ��ͷе㣬�����¶ȹ��ߣ����¼��屽��ȩ��������

����Ŀ����ѧ��Ӧ������ͨ��ʵ��ⶨ�ģ����л�ѧ��Ӧ���ʵIJ����У��������ݲ����е���( )
ѡ�� | ��ѧ��Ӧ | ��������(��λʱ����) |
A | 2NO2 | ��ɫ��dz |
B | Zn+H2SO4=ZnSO4+H2 | H2��� |
C | CO(g)+H2O(g)=CO2(g)+H2(g) | ѹǿ�仯 |
D | Ca(OH)2+Na2CO3=CaCO3��+2NaOH | �������� |
����Ŀ��������Ԫ��W��X��Y��Z��ԭ�����������������в���Ԫ�������ڱ��е�λ����ͼ��ʾ��һ��WX2�����к���22�����ӣ�Y����������X��Z�ĺ˵����֮�͵ġ��롣����˵����ȷ����
W | X | |
Z |
A. �ǽ����ԣ�W < Z
B. �����ӵİ뾶��X2- < Y2+
C. �е㣺H2X < H2Z
D. WX2�ĵ���ʽΪ��![]()