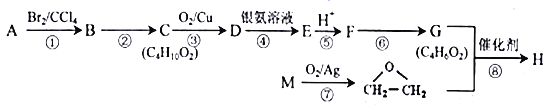
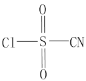
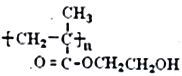��Ŀ����
����Ŀ��I.��1��ֱ���ŷ�úȼ�ղ������������������صĻ������⡣
�ټ��������Ը��������Һ����úȼ�ղ�����SO2���ù����и����������ԭΪMn2+����д���ù��̵����ӷ���ʽ______________��
�ڽ�ȼú�����Ķ�����̼���Ի��գ��ɽ���̼���ŷš���ͼ��ͨ���˹�������ã���CO2��H2OΪԭ���Ʊ�HCOOH��O2��ԭ��ʾ��ͼ��a�缫���ƣ�_____________(�������������)��b�缫�ķ�Ӧʽ��________________________��

��2���������NaClO��Ca��ClO��2�����ռ���Ҳ�ܵõ��Ϻõ���������Ч����
��֪���з�Ӧ��
SO2(g)+2OH��(aq) ==SO32��(aq)+H2O(l) ��H1
ClO��(aq)+SO32��(aq) ==SO42��(aq)+Cl��(aq) ��H2
CaSO4(s)==Ca2+��aq��+SO42����aq�� ��H3
��ӦSO2(g)+ Ca2+��aq��+ ClO��(aq) +2OH��(aq) = CaSO4(s) +H2O(l) +Cl��(aq)�Ħ�H=_____��
II.��3��FeO42����ˮ��Һ�еĴ�����̬��ͼ��ʾ��

������pH��10��������Һ�м�������pH��2��HFeO4���ķֲ������ı仯�����__________��
������pH��6��������Һ�еμ�KOH��Һ������Һ�к���Ԫ�ص����У�_________ת��Ϊ_________(��������)��
���𰸡�2MnO4��+5SO2+2H2O=2Mn2++5SO42��+4H+ ����CO2+2e��+2H+=HCOOH��H1+��H2-��H3 ��������СHFeO4��FeO42��
��������
(1)�������Ը��������Һ����úȼ�ղ�����SO2�������и����������ԭΪMn2������SO2������ΪSO42�����ݴ�д����Ӧ�����ӷ���ʽ��
��a�缫����H2Oת��ΪO2���˹���Ϊʧȥ���ӵķ�Ӧ��ԭ�����ʧȥ���ӵĵ缫Ϊ������b�缫Ϊ������CO2��H���õ����ӣ�����HCOOH��
(2)���ݸ�˹���ɽ��⡣
��3���ٸ���ͼƬ֪�����ݲ�ͬpHʱ��HFeO4���ı仯ͼ���жϣ�����pH=6��������Һ�м�KOH��Һ��������Ӧ�����ӷ���ʽΪ��HFeO4��+OH��=FeO42��+H2O��
(1)���������Ը��������Һ����úȼ�ղ�����SO2�������и����������ԭΪMn2������SO2������ΪSO42������ù��̵����ӷ���ʽΪ��5SO2+2MnO4��+2H2O�T2Mn2��+5SO42��+4H����
��a�缫����H2Oת��ΪO2���˹���Ϊʧȥ���ӵķ�Ӧ��ԭ�����ʧȥ���ӵĵ缫Ϊ������b�缫Ϊ������CO2��H���õ����ӣ�����HCOOH����b�缫�ķ�Ӧʽ��CO2+2H��+2e���THCOOH
��2�����ݸ�˹���ɣ���SO2(g)+2OH��(aq) ==SO32��(aq)+H2O(l) ��H1
��ClO��(aq)+SO32��(aq) ==SO42��(aq)+Cl��(aq) ��H2
��CaSO4(s)==Ca2+��aq��+SO42����aq�� ��H3
���+��-�۵÷�ӦSO2(g)+ Ca2+��aq��+ ClO��(aq) +2OH��(aq) = CaSO4(s) +H2O(l) +Cl��(aq)�Ħ�H= ��H1+��H2-��H3 ��
��3����ͼ�������֪������pH��10��������Һ�м�������pH��2��HFeO4���ķֲ������ı仯�������������С������pH=6��������Һ�м�KOH��Һ��������Ӧ�����ӷ���ʽΪ��HFeO4��+OH��=FeO42��+H2O��HFeO4��ת��ΪFeO42����

 ��У����ϵ�д�
��У����ϵ�д�