��Ŀ����
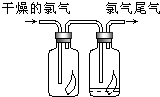 ��1����ͼ��ʾ������������ͨ��ʢ�и�����ɫ�����Ĺ��ƿ��ʢ�г�ʪ��ɫ�����Ĺ��ƿ���ɹ۲쵽��������
��1����ͼ��ʾ������������ͨ��ʢ�и�����ɫ�����Ĺ��ƿ��ʢ�г�ʪ��ɫ�����Ĺ��ƿ���ɹ۲쵽���������������ɫ��������������ʪ����ɫ������ɫ
�������ɫ��������������ʪ����ɫ������ɫ
����2��Ϊ�˷�ֹ����β����Ⱦ����������
NaOH
NaOH
��Һ���ն����������ԭ���ǣ��û�ѧ����ʽ��ʾ��Cl2+2NaOH=NaCl+NaClO+H2O
Cl2+2NaOH=NaCl+NaClO+H2O
����������1��������Ư���ԣ�������ˮ��Ӧ���ɵĴ��������Ư���ԣ�
��2����������������������Һ��Ӧ������β����
��2����������������������Һ��Ӧ������β����
����⣺��1��������Ư���ԣ�������ˮ��Ӧ���ɵĴ��������Ư���ԣ�Cl2+H2O=HCl+HClO���������ɫ��������������ʪ����ɫ������ɫ��
�ʴ�Ϊ���������ɫ��������������ʪ����ɫ������ɫ��
��2��Ϊ�˷�ֹ����β����Ⱦ����������NaOH��Һ���գ��÷�Ӧ�Ļ�ѧ����ʽΪCl2+2NaOH=NaCl+NaClO+H2O���ʴ�Ϊ��NaOH��Cl2+2NaOH=NaCl+NaClO+H2O��
�ʴ�Ϊ���������ɫ��������������ʪ����ɫ������ɫ��
��2��Ϊ�˷�ֹ����β����Ⱦ����������NaOH��Һ���գ��÷�Ӧ�Ļ�ѧ����ʽΪCl2+2NaOH=NaCl+NaClO+H2O���ʴ�Ϊ��NaOH��Cl2+2NaOH=NaCl+NaClO+H2O��
���������⿼����������ѧ���ʵ�Ӧ�á������Ĵ�����������ȷ����Ư���Ե��Ǵ������ǽ����Ĺؼ�����Ŀ�ϼ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
 ��1����ͼ��ʾ������������ͨ��ʢ�и�����ɫ�����Ĺ��ƿ��ʢ�г�ʪ��ɫ�����Ĺ��ƿ���ɹ۲쵽��������
��1����ͼ��ʾ������������ͨ��ʢ�и�����ɫ�����Ĺ��ƿ��ʢ�г�ʪ��ɫ�����Ĺ��ƿ���ɹ۲쵽�������� ��1����ͼ��ʾ������������ͨ��ʢ�и�����ɫ�����Ĺ��ƿ��ʢ�г�ʪ��ɫ�����Ĺ��ƿ���ɹ۲쵽��������
��1����ͼ��ʾ������������ͨ��ʢ�и�����ɫ�����Ĺ��ƿ��ʢ�г�ʪ��ɫ�����Ĺ��ƿ���ɹ۲쵽�������� ��1����ͼ��ʾ������������ͨ��ʢ�и�����ɫ�����Ĺ��ƿ��ʢ�г�ʪ��ɫ�����Ĺ��ƿ���ɹ۲쵽��������
��1����ͼ��ʾ������������ͨ��ʢ�и�����ɫ�����Ĺ��ƿ��ʢ�г�ʪ��ɫ�����Ĺ��ƿ���ɹ۲쵽��������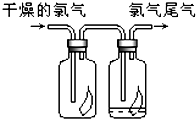 ��1����ͼ��ʾ������������ͨ��ʢ�и�����ɫ�����Ĺ��ƿ��ʢ�г�ʪ��ɫ�����Ĺ��ƿ���ɹ۲쵽������
��1����ͼ��ʾ������������ͨ��ʢ�и�����ɫ�����Ĺ��ƿ��ʢ�г�ʪ��ɫ�����Ĺ��ƿ���ɹ۲쵽������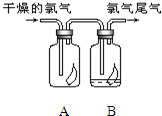 ��1����ͼ��ʾ������������ͨ��ʢ�и�����ɫ�����Ĺ��ƿ��ʢ�г�ʪ��ɫ�����Ĺ��ƿ���ɹ۲쵽��������
��1����ͼ��ʾ������������ͨ��ʢ�и�����ɫ�����Ĺ��ƿ��ʢ�г�ʪ��ɫ�����Ĺ��ƿ���ɹ۲쵽��������