��Ŀ����
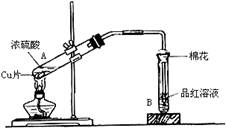
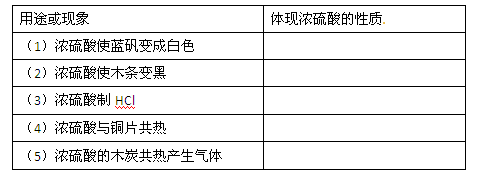
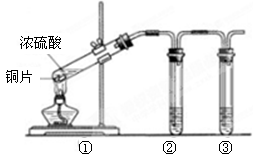
(10��) ijͬѧ����ͼ��ʾװ��̽��SO2�����ʼ����й�ʵ�飮
(1)ʵ�������������ƹ����һ��Ũ�ȵ����ᷴӦ�Ʊ�SO2���壬д���÷�Ӧ�Ļ�ѧ����ʽ______________________________________________________________
(2)�ֱ�SO2����ͨ������C��Һ�У���ش��������⣺
������SO2ͨ����ɫʯ����Һ��������______________������ͨ�����SO2���壬������________________��
��SO2ͨ����ɫKMnO4��Һ��������______________���䷴Ӧ�����ӷ���ʽΪ_______________________��
�۹���SO2������ͨ�����ʯ��ˮ�У�����___________________________________
����CΪ˫��ˮ����ͨ��SO2������ж�������Һ��________(�����ʵĻ�ѧʽ)������ͬѧ�Ʊ���SO2�����л���CO2���壬�������ʵ�ԭ��������������ƹ����л���__________��

(1)ʵ�������������ƹ����һ��Ũ�ȵ����ᷴӦ�Ʊ�SO2���壬д���÷�Ӧ�Ļ�ѧ����ʽ______________________________________________________________
(2)�ֱ�SO2����ͨ������C��Һ�У���ش��������⣺
������SO2ͨ����ɫʯ����Һ��������______________������ͨ�����SO2���壬������________________��
��SO2ͨ����ɫKMnO4��Һ��������______________���䷴Ӧ�����ӷ���ʽΪ_______________________��
�۹���SO2������ͨ�����ʯ��ˮ�У�����___________________________________
����CΪ˫��ˮ����ͨ��SO2������ж�������Һ��________(�����ʵĻ�ѧʽ)������ͬѧ�Ʊ���SO2�����л���CO2���壬�������ʵ�ԭ��������������ƹ����л���__________��
(1)Na2SO3��H2SO4===Na2SO4��SO2����H2O
(2)�ٱ�죨1�֣�������ɫ������
����ɫ�䵭����ɫ��ʧ 5SO2��2MnO��2H2O===5SO��2Mn2����4H��
���ȱ���ǣ����ֱ����
��H2SO4 ��̼���λ�̼������
(2)�ٱ�죨1�֣�������ɫ������
����ɫ�䵭����ɫ��ʧ 5SO2��2MnO��2H2O===5SO��2Mn2����4H��
���ȱ���ǣ����ֱ����
��H2SO4 ��̼���λ�̼������
�����������1��ʵ�����Ʊ�SO2�ķ���ʽΪ��Na2SO3��H2SO4===Na2SO4��SO2����H2O
��2����SO2��Ȼ��Ư���ԣ����Dz���Ư��ʯ�ֻ��ʹʯ���첻����ɫ��
��SO2����ǿ��ԭ������ͨ�뵽KMnO4��Һ�У�KMnO4��Һ��ɫ��ȥ����Ӧ�����ӷ���ʽΪ��5SO2��2MnO��2H2O===5SO��2Mn2����4H����
��CaSO3����Ca(HSO3)2���ܣ�����SO2ͨ�����ʯ��ˮ�в�������������ͨ�룬�����ܽ⡣
��H2O2���������ԣ�SO2���л�ԭ�ԣ����Խ�SO2ͨ�뵽H2O2���ܷ�Ӧ����H2SO4������Ƶõ�SO2����CO2���ʣ�˵��Na2SO3�л�����Na2CO3��NaHCO3��2������
����������dz���������Ҫ����ѧ����SO2���ʵ����ա�

��ϰ��ϵ�д�
�����Ŀ

 H2SO4
H2SO4 SO2
SO2 H2SO4
H2SO4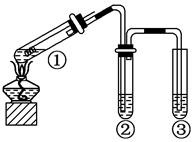
 Cu2�� + 2H2O + SO2��
Cu2�� + 2H2O + SO2��