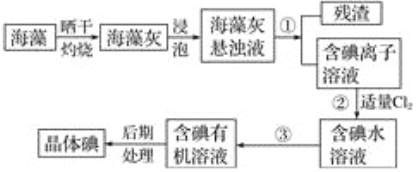
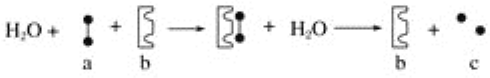��Ŀ����
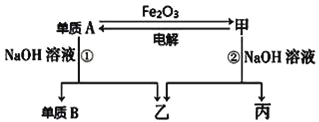
����Ŀ���й����ʵ�ת����ϵ����ͼ��ʾ��A��C��E�dz����Ľ������ʣ�EΪ�Ϻ�ɫ����Ӧ�ٿ����ں������죬B�dz��������Ҫ�ɷ֣�F����Һ�м���KSCN��Һ��졣�����ַ�Ӧ���������ʡ�ԣ�
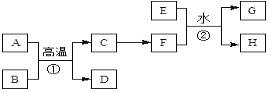
��ش��������⣺
��1��D�Ļ�ѧʽ��____��
��2����ѡ��____�����ĸ����ʵ�ַ�ӦC��F��ת����
a��ϡ���� b������/��ȼ c��CuSO4��Һ
��3����Ӧ�ٵĻ�ѧ����ʽΪ____��
��4����Ӧ�ڵ����ӷ���ʽΪ____��
���𰸡�Al2O3 b 2Al+Fe2O3![]() 2Fe + Al2O3 Cu + 2Fe3+�� Cu2+ + 2Fe2+
2Fe + Al2O3 Cu + 2Fe3+�� Cu2+ + 2Fe2+
��������
�ɷ�Ӧ�ٿ����ں������죬B�dz��������Ҫ�ɷ֣������ƲⷴӦ��Ϊ���ȷ�Ӧ��AΪ����BΪ������������ CΪ����DΪ������������EΪ�Ϻ�ɫ��������Cu��F����Һ�м���KSCN��Һ��죬ȷ��F��Һ���������ӡ�
��1�������Ϸ�����֪D�Ļ�ѧʽ��Al2O3��
��ȷ�𰸣� Al2O3��
��2����������ֱ���������Σ�����ѡ��ǿ����������ѡb��
��ȷ�𰸣� b��
��3����Ӧ�����ȷ�Ӧ����ѧ����ʽ2Al+Fe2O3![]() 2Fe + Al2O3��
2Fe + Al2O3��
��ȷ�𰸣� 2Al+Fe2O3![]() 2Fe + Al2O3��
2Fe + Al2O3��
��4����Ӧ�ڵ����ӷ���ʽΪCu + 2Fe3+�� Cu2+ + 2Fe2+��
��ȷ�𰸣� Cu + 2Fe3+�� Cu2+ + 2Fe2+��

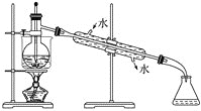
����Ŀ��ͨ����̼�ڸ����»�ԭ���������Ƶôֹ裬�ֹ�(������������������)��������Ӧ�������Ȼ���(��Ӧ�¶�450-500��)���������辭�ᴿ����������ԭ�ɵøߴ��衣������ʵ�����Ʊ����Ȼ����װ��ʾ��ͼ��(D��Ӳ�ʲ�������ʢװ�ֹ�)
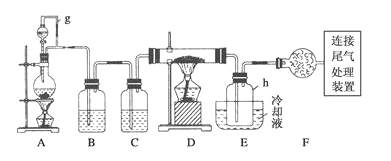
�����Ϣ����:a.����������ˮ����Ӧ���ɹ�����Ȼ��⣻
b.�������������ڸ����¾���������ֱ�ӷ�Ӧ������Ӧ���Ȼ��
c.�й����ʵ������������±�:
���� | SiCl4 | BCl3 | AlCl3 | FeCl3 | PCl5 |
�е�/�� | 57.7 | 12.8 | �� | 315 | �� |
�۵�/�� | ��70.0 | ��107.2 | �� | �� | �� |
�����¶�/�� | �� | �� | 180 | 300 | 162 |
��ش���������:
(1)д����̼�ڸ����»�ԭ���������Ƶôֹ�Ļ�ѧ����ʽ_________________________��
(2)д��װ��A�з�����Ӧ�����ӷ���ʽ__________________________________��
(3)װ��A��g�ܵ�������_________________��װ��C�е��Լ���____________��
(4)װ��E��hƿ�ռ����Ĵֲ����ͨ���������õ��ߴ������Ȼ��裬�����IJ������У�����Ԫ������ܻ����е�����Ԫ����________(��дԪ�ط���)��
(5)д��β������װ���з�����Ӧ�����ӷ���ʽ___________________________��
(6)����ʵ��װ������һ���ԵIJ���֮�����������ĸĽ�����������: ___________��