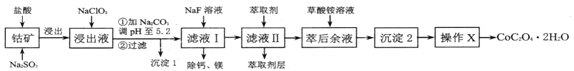
��Ŀ����
����Ŀ��±��Ԫ�صĵ��ʺͻ���������������������Ҫ����;��
��1����ԭ�ӵĺ�������Ų�ʽΪ[Ar]__________����_____��δ�ɶԵ��ӡ�
��2����һ��Ũ�ȵ� HF ��Һ�У����������Ե���ʽ(HF)2 ���ڵġ�ʹ��������ӵϵ���������____________��
��3�������±����ݣ������۵��Ӳ�ȱ仯��ԭ��________________��

��4��HIO3 ������_____���ǿ�ڡ������ڡ���HIO4��ԭ����_________________��
��5��ClO2������ԭ�ӵ��ӻ�����Ϊ________��ClO3�Ŀռ乹��Ϊ__________��
��6����������������Ҫ�أ�
��ԭ�������������ʾ�����ڲ����������λ�á���ͼ�� CaF2 �ľ���������ԭ��������� A ��Ϊ(0��0��0)��B ��Ϊ(![]() ��
�� ![]() ��0)��C ��Ϊ(1��1��1)���� D �������������Ϊ__________��
��0)��C ��Ϊ(1��1��1)���� D �������������Ϊ__________��
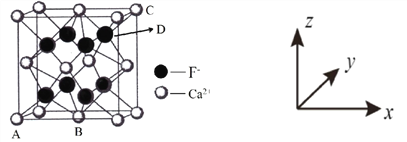
�ھ������������������Ĵ�С����״����֪ CaF2 ������ܶ�Ϊ c gcm3������ Ca2+����������� F֮��ľ���Ϊ_________ nm���� NA Ϊ�����ӵ�������ֵ���ú� c��NA ��ʽ�ӱ�ʾ����
���𰸡� 3d104s24p5 1 ��� ���Ӿ��������Ӱ뾶ԽС�����������ĵ��Խ�࣬�����ܾ�Խ������۵�Խ�ߣ�Ħ��Ӳ��Խ�� ���� HIO4�����е�ļ�̬��HIO3�и��ߣ�����I-O-H��O�ĵ�����Iƫ�Ƶø��࣬��ˮ���ӵ������£����������H+�������Ը�ǿ sp3 ������ ��![]() ��
�� ![]() ��
�� ![]() ��
�� ![]()
��������(1)��Ϊ35��Ԫ�أ���ԭ�ӵĺ�������Ų�ʽΪ[Ar] 3d104s24p5��4p5����1��δ�ɶԵ��ӣ��ʴ�Ϊ��3d104s24p5 ��1��
(2)��������Ӽ������γ��������һ��Ũ�ȵ� HF ��Һ�У����������Ե���ʽ(HF)2 ���ڵġ�ʹ��������ӵϵ���������������ʴ�Ϊ�������
(3)���Ӿ��������Ӱ뾶ԽС�����������ĵ��Խ�࣬�����ܾ�Խ������۵�Խ�ߣ�Ħ��Ӳ��Խ�����־����У������Ӱ뾶�������Ӱ뾶С�������Ƶ��۵��Ӳ�ȱ��Ȼ��Ƹߣ�þ���Ӱ뾶�ȸ����Ӱ뾶С������þ���۵��Ӳ�ȱ������Ƹߣ�����þ�������������Ӵ�2����λ��ɣ��������ƺ��Ȼ��������Ӵ�1����λ��ɣ��������þ�������Ƶ��۵��Ӳ�ȱȷ����ƺ��Ȼ��Ƹߣ��ʴ�Ϊ�����Ӿ��������Ӱ뾶ԽС�����������ĵ��Խ�࣬�����ܾ�Խ������۵�Խ�ߣ�Ħ��Ӳ��Խ��
(4)HIO4�����е�ļ�̬��HIO3�и��ߣ�����I-O-H��O�ĵ�����Iƫ�Ƶø��࣬��ˮ���ӵ������£����������H+�������Ը�ǿ���ʴ�Ϊ�����ڣ�HIO4�����е�ļ�̬��HIO3�и��ߣ�����I-O-H��O�ĵ�����Iƫ�Ƶø��࣬��ˮ���ӵ������£����������H+�������Ը�ǿ��
(5)ClO2������ԭ�ӵļ۲���Ӷ���=2+![]() ��(7+1-2��2)=4������sp3�ӻ���ClO3������ԭ�ӵļ۲���Ӷ���=3+
��(7+1-2��2)=4������sp3�ӻ���ClO3������ԭ�ӵļ۲���Ӷ���=3+![]() ��(7+1-2��3)=4������sp3�ӻ����ռ乹��Ϊ�����Σ��ʴ�Ϊ��sp3 �� �����Σ�
��(7+1-2��3)=4������sp3�ӻ����ռ乹��Ϊ�����Σ��ʴ�Ϊ��sp3 �� �����Σ�
(6)������ԭ��������� A ��Ϊ(0��0��0)��˵��AΪ����ԭ�㣬B ��Ϊ(![]() ��
�� ![]() ��0)����D��C ��Ϊ(1��1��1)����D�������������Ϊ(
��0)����D��C ��Ϊ(1��1��1)����D�������������Ϊ(![]() ��
�� ![]() ��
�� ![]() ) ���ʴ�Ϊ��(
) ���ʴ�Ϊ��(![]() ��
�� ![]() ��
�� ![]() )��
)��
��CaF2�����У��������ĿΪ8��![]() +6��
+6��![]() =4������ȫ���ھ����ڲ�����ĿΪ8����ͼ�а����Ǹ����ӣ��Ѿ����ֳ�8��С����������ͼ
=4������ȫ���ھ����ڲ�����ĿΪ8����ͼ�а����Ǹ����ӣ��Ѿ����ֳ�8��С����������ͼ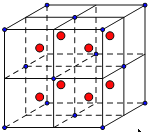 ����F-λ��С����������ģ���С������Ca2+��F-֮����������ΪС���������Խ��ߵ�һ�롣CaF2 ������ܶ�Ϊ c gcm3��1mol����������Ϊ4��78g�����ı߳�Ϊ
����F-λ��С����������ģ���С������Ca2+��F-֮����������ΪС���������Խ��ߵ�һ�롣CaF2 ������ܶ�Ϊ c gcm3��1mol����������Ϊ4��78g�����ı߳�Ϊ cm=
cm= ��107 nm�����Ca2+��F-֮����������Ϊ
��107 nm�����Ca2+��F-֮����������Ϊ![]() ��
�� ��107 nm
��107 nm
�ʴ�Ϊ�� ![]() ��
��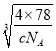 ��107��
��107��

 �����������Ů��ͯ������ϵ�д�
�����������Ů��ͯ������ϵ�д�����Ŀ��CH3ClΪ��ɫ���Դ���ζ�����壬�ܶ�Ϊ2.25g/L���۵�Ϊ-24.2����20��ʱ��ˮ�е��ܽ��Ϊ400mL���������Ҵ��ͱ������л��ܼ���
����ʵ������ȡCH3Cl��ԭ����CH3OH +HCl(Ũ)![]() CH3Cl+H2O�����岽�����£�
CH3Cl+H2O�����岽�����£�
������ZnCl2���壻
����ȡ24g��ϸ����ˮZnCl2����ȡ20mLŨ�������Բ����ƿ��
ͬʱ��ȡһ�����ļ״������Һ©���У�
������Һ©����ļ״���ε�����ƿ�в����ȣ���ZnCl2��ȫ�ܽ����CH3Cl�����ݳ���������ˮ���ռ���

��ش�
��1��ʵ���Ҹ���ZnCl2�����Ƶ���ˮZnCl2�ķ����� ��
��2����Ӧ�����е�����ƿ�м״�������������٣��״���Ũ��������ʵ���Ũ�Ƚӽ����������� ��
��3��ʵ����Ϊ������ˮ���ռ�CH3Cl�� ��
������ij���ϼ��أ�CH4�����е�һ��Hԭ�ӱ�Clԭ��ȡ�������ȶ����ܵ�Ӱ�죬�ɱ�ǿ���������Ը������������������ֻϴ��ƿ���ֱ�ʢ�������Լ���
A��1.5%KMnO4(H+)��Һ�� | B������ˮ�� | C��5%Na2SO3��Һ�� | D��98%H2SO4�� |
��1��Ϊ֤ʵ��һ���۵Ŀɿ��ԣ��������ѡ����ǡ����ϴ��ƿ����aװ�����ɵ���������ͨ��ϴ��ƿ ����ϴ��ƿ��ţ�������۲쵽 ��֤ʵ�������ϵ���ȷ�ԡ�
��2��д��ϴ��ƿ�з�����Ӧ�����ӷ���ʽ(CԪ�ص���������ΪCO2)��
��3�����CH3Cl�Ǵ�������Ⱦ�����ϴ��ƿ֮��Ӧ��һֻʢ ��ϴ��ƿ��