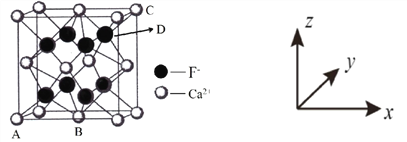
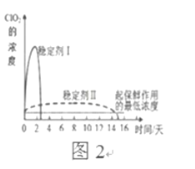
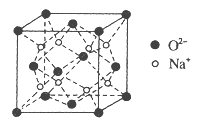
��Ŀ����
����Ŀ��CH3ClΪ��ɫ���Դ���ζ�����壬�ܶ�Ϊ2.25g/L���۵�Ϊ-24.2����20��ʱ��ˮ�е��ܽ��Ϊ400mL���������Ҵ��ͱ������л��ܼ���
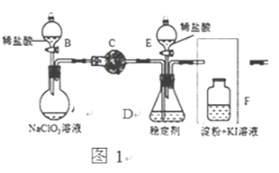
����ʵ������ȡCH3Cl��ԭ����CH3OH +HCl(Ũ)![]() CH3Cl+H2O�����岽�����£�
CH3Cl+H2O�����岽�����£�
������ZnCl2���壻
����ȡ24g��ϸ����ˮZnCl2����ȡ20mLŨ�������Բ����ƿ��
ͬʱ��ȡһ�����ļ״������Һ©���У�
������Һ©����ļ״���ε�����ƿ�в����ȣ���ZnCl2��ȫ�ܽ����CH3Cl�����ݳ���������ˮ���ռ���

��ش�
��1��ʵ���Ҹ���ZnCl2�����Ƶ���ˮZnCl2�ķ����� ��
��2����Ӧ�����е�����ƿ�м״�������������٣��״���Ũ��������ʵ���Ũ�Ƚӽ����������� ��
��3��ʵ����Ϊ������ˮ���ռ�CH3Cl�� ��
������ij���ϼ��أ�CH4�����е�һ��Hԭ�ӱ�Clԭ��ȡ�������ȶ����ܵ�Ӱ�죬�ɱ�ǿ���������Ը������������������ֻϴ��ƿ���ֱ�ʢ�������Լ���
A��1.5%KMnO4(H+)��Һ�� | B������ˮ�� | C��5%Na2SO3��Һ�� | D��98%H2SO4�� |
��1��Ϊ֤ʵ��һ���۵Ŀɿ��ԣ��������ѡ����ǡ����ϴ��ƿ����aװ�����ɵ���������ͨ��ϴ��ƿ ����ϴ��ƿ��ţ�������۲쵽 ��֤ʵ�������ϵ���ȷ�ԡ�
��2��д��ϴ��ƿ�з�����Ӧ�����ӷ���ʽ(CԪ�ص���������ΪCO2)��
��3�����CH3Cl�Ǵ�������Ⱦ�����ϴ��ƿ֮��Ӧ��һֻʢ ��ϴ��ƿ��
���𰸡���.(1)�ڸ����HCl�����м��ȣ� (2)���������������״���ת���ʣ�(3)CH3Cl��������ˮ������ˮ���ɳ�ȥHCl��CH3OH���������壻������1��BAC ��A��ɫ��ȥ����2��10CH3Cl��14MnO4����42H����14Mn2���� 10CO2����5Cl2����36H2O��SO32����Cl2��H2O��SO42����2Cl����H������3���Ҵ�(�������л��ܼ�)
�����������������������1����ΪZnCl2Ϊǿ�������Σ�����ˮ�ⷴӦ����HCl��Ϊ�˷�ֹZnCl2��ˮ�⣬����ʵ���Ҹ���ZnCl2�����Ƶ���ˮZnCl2�ķ������ڸ����HCl�����м��ȡ�
��2���������������ƽ��������Ӧ�����ƶ�������״���ת���ʡ�
��3��CH3Cl��20��ʱ��ˮ�е��ܽ��Ϊ400mL����������ˮ������ˮ���ɳ�ȥHCl��CH3OH���������塣
������1��ʵ������ȡCH3Cl��ԭ����CH3OH +HCl��Ũ��![]() CH3Cl+H2O������ȡ��CH3Cl�к���HCl��������ͨ������ˮ����ȥHCl���ʣ���ͨ��1.5%KMnO4��H+����Һ����֤CH3Cl�ܷ����Ը��������Һ�����������5%Na2SO3��Һ�������ɵ��ж�����Cl2�����Խ�aװ�����ɵ���������BAC������۲쵽A����ɫ���Ը��������Һ��ɫ��˵��CH3Cl�ܱ����Ը��������Һ������
CH3Cl+H2O������ȡ��CH3Cl�к���HCl��������ͨ������ˮ����ȥHCl���ʣ���ͨ��1.5%KMnO4��H+����Һ����֤CH3Cl�ܷ����Ը��������Һ�����������5%Na2SO3��Һ�������ɵ��ж�����Cl2�����Խ�aװ�����ɵ���������BAC������۲쵽A����ɫ���Ը��������Һ��ɫ��˵��CH3Cl�ܱ����Ը��������Һ������
��2��CH3Clͨ�����Ը��������Һ����������ԭ��Ӧ��CH3Cl������ΪCO2��Cl2��������MnO4����ԭΪMn2+�����ݵ���غ��Ԫ���غ㣬��Ӧ���л���H+���������л���H2O�����ݻ��ϼ����ߵ��ܼ������ڽ��͵��ܼ�����ƽ�ɵ����ӷ���ʽΪ10CH3Cl��14MnO4����42H����14Mn2���� 10CO2����5Cl2����36H2O���ж���Cl2��Na2SO3���գ����ӷ���ʽΪSO32����Cl2��H2O��SO42����2Cl����H����
��3����ΪCH3Cl�������Ҵ��ͱ������л��ܼ����������CH3Cl�Ǵ�������Ⱦ�����ϴ��ƿ֮��Ӧ��һֻʢ�Ҵ����������л��ܼ�����ϴ��ƿ����������CH3Cl��

����Ŀ��(1)�Ӵ��������Ṥ���У�������Ӧ���ں��ݡ��¶�Ϊ450�沢�д������ڵ������½��У�
2SO2(g)+O2(g) ![]() 2SO3(g) ��H=-190Kj/mol
2SO3(g) ��H=-190Kj/mol
��������������˵��������Ӧ�Ѵ�ƽ�����____________��
a. ![]() (O2)��=2
(O2)��=2![]() (SO3)�� b.������������ܶȲ���ʱ����仯
(SO3)�� b.������������ܶȲ���ʱ����仯
c.�����������ƽ����Է�����������ʱ����仯 d.����������ķ�����������ʱ����仯
����һ���̶��ݻ�Ϊ5L���ܱ������г���0.20molSO2��0.10molO2������Ӻ�ﵽƽ�⣬��������к�SO30.18mol����![]() (O2)=__________mol��L-1��min-1��������ͨ��0.40 mol SO2��0.20molO2��ƽ
(O2)=__________mol��L-1��min-1��������ͨ��0.40 mol SO2��0.20molO2��ƽ
��______�ƶ����������Ӧ���������淴Ӧ�����������ٴδﵽƽ���________mol3) <_____mol��
(2)��ҵ�������ص�ԭ������NH3��CO2Ϊԭ�Ϻϳ�����[CO(NH2)2]����Ӧ�Ļ�ѧ����ʽΪ2NH3 (g)+ CO2(g) ![]() CO(NH2)2(1)+H2O(1)���÷�Ӧ��ƽ�ⳣ�����¶ȹ�ϵ���£�
CO(NH2)2(1)+H2O(1)���÷�Ӧ��ƽ�ⳣ�����¶ȹ�ϵ���£�
T/�� | 165 | 175 | 185 | 195 |
K | 111.9 | 74.1 | 50.6 | 34.8 |
�٦�H_______0���>������<����=����
����һ���¶Ⱥ�ѹǿ�£���ԭ�����е�NH3��CO2�����ʵ���֮�ȣ���̼�ȣ�![]() =x����ͼ�ǰ�̼�ȣ�x����CO2ƽ��ת���ʣ������Ĺ�ϵ��������x����������ԭ����___________��
=x����ͼ�ǰ�̼�ȣ�x����CO2ƽ��ת���ʣ������Ĺ�ϵ��������x����������ԭ����___________��

����ͼ�е�B���Ӧ��NH3��ƽ��ת����Ϊ___________��