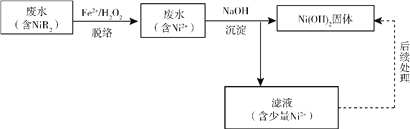
��Ŀ����
����Ŀ����. �ס��ҡ��������ֲ�����ͬ���ӵĿ�����ǿ����ʣ�������������������ʾ��
������ | NH4+��Mg2+��Ba2+ |
������ | OH����NO3����Cl�� |
ȡ�����������ֻ�����������ͬ�������Һ�������ʵ����ʵ���Ũ�ȣ�c(��)>c(��)>c(��)��
��1������_________________
��2��������__________________�����ʵ��ȷ�����������_______________________���������������ȷ������˿ղ��
��. ��ͼΪʵ����ijŨ�����Լ�ƿ�ı�ǩ���Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺
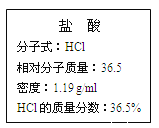
��1����Ũ������HCl�����ʵ���Ũ��Ϊ__________��
��2��ijѧ����������Ũ���������ˮ����500 mL���ʵ���Ũ��Ϊ0.40 mol/L��ϡ���ᡣ
�ٸ�ѧ������Ͳ��ȡ________mL����Ũ����������ơ�
�������ƹ����У�����ʵ��������������������ʵ���Ũ���к�Ӱ�죿���ڿո����� ��ƫ�ߡ�����ƫ�͡�����Ӱ�족����
����ʱ���ӹ۲�_________��
���ݺ���ҡ�ȡ����ú���Һ���½����ټ�����������ˮ__________��
���𰸡�Ba(OH)2 NH4NO3��NH4Clȡ��������Һ���Թ��У������еμ���������������Һ�����а�ɫ�������ɣ����ΪNH4Cl����֮��ΪNH4NO311.9mol/L16.8mLƫ��ƫС
��������
�ס��ҡ������ǿ�����ǿ����ʣ���OH-ֻ����Na+���NaOH�����ֻ����ﲻ����ͬ���ӣ����Լס��ҡ�����������������ϣ���һ�飺NaOH����NH4��2SO4��Mg��NO3��2���ڶ��飺NaOH��NH4NO3��MgSO4����������������ȣ���Һ�������Լ����ʵ���Ũ�ȴ�С�жϣ���Է���������Mr��������Mr���ң���Mr���ף�����.������Һ��1L������c=n/V���㣻����ϡ�Ͷ��ɼ��㣬ϡ��ǰ�����ʵ����ʵ������䣻����c=n/V������������
��1�����ڼס��ҡ��������ֲ�����ͬ���ӵĿ�����ǿ����ʣ���NH4+��Mg2+��Ӧ�������Ӿ�������OH-����OH-��Ӧ����������Ba2+����������ΪBa��OH��2���������ֻ��������ΪNH4NO3��MgCl2��NH4Cl��Mg��NO3��2��ȡ�����������ֻ�����������ͬ�������Һ�����������ʵ���Ũ�ȣ�c���ף���c���ң���c����������Ħ������M���ף���M���ң���M����������Ħ������������Ba��OH��2���ʱ�ΪBa��OH��2����2������ΪNH4NO3��MgCl2��NH4Cl��Mg��NO3��2������Ħ��������С���ʼ���Ϊ��NH4NO3��NH4Cl����NH4NO3��NH4Cl�������Ӳ�ͬ���ʼ�ȷ���ķ�����ȡ��������Һ���Թ��У������еμ���������������Һ�����а�ɫ�������ɣ���֤��ΪNH4Cl����֮��ΪNH4NO3��
��.��1�����������Ϊ1L��c��HCl��=1L��1000��1.19g/L��36.5%��36.5g/mol
��1L=11.9molL1����2��������Ҫ��Ũ��������ΪVL����0.5L��0.400molL1=
V��11.9molL1���ɵ�V=0.0168L=16.8mL���ڶ���ʱ��������ƿ�̶��ߣ�������Ƶ���Һ���ƫС��Ũ��ƫ��ˮʱ�����̶��ߣ��ý�ͷ�ι�������������ʵ����ʵ���ƫС��Ũ��ƫС��

 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�