��Ŀ����
����Ŀ����ͼ���������������л��������빦�ܹ�ϵͼ����ͼ�Ƕ��ǵ����ͼ�����ͼ�ش��������⡣
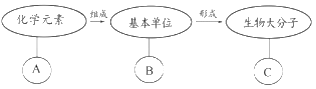
��1������ϸ���������Ԫ����_____________���������ں��������л���������ͼ�е�_______������ĸ��
��2����ͼ������D�Ļ���Ԫ�������________������C�IJ�ͬȡ����_________�IJ�ͬ��
��3��С������ϸ���У�����E��ָ__________________��
��4����ͬ������E��F���������ֽ⣬�������϶����_____________��
��5����ͼB��C�����ĵ��Ƿֱ���_______��___________(��д��������)������ˮ���������_____�ǣ����ɵ��ۡ���ά�ء���ԭ�ȶ��ǵĵ��嶼��____________________��
��6�����ۡ���ѿ�ǡ������ǡ���ԭ������ij�������������ijЩ�仯��������һ����________(������ֲ�)��
���𰸡�C G C H O N P R�� ���� F ���� ������ ���� ������ ����
��������
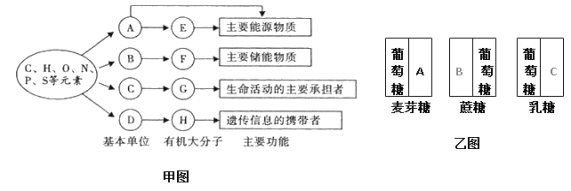
����ͼ��֪����ͼ�Ǻ��ᡢ�����ʡ�֬������������Ԫ�ء�������λ�����ࡢ�ֲ����ܣ�����E��A����Ҫ����Դ���ʣ������࣬E��A��ɣ�A�������ǣ�E�ǵ��ۻ���ԭ��F����Ҫ�Ĵ������ʣ�Ϊ֬����B��֬���Ļ�����ɵ�λ���ͺ�֬���G�����������Ҫ�е��ߣ�Ϊ�����ʣ�C�ǵ����ʵĻ�����ɵ�λ�����H���Ŵ���Ϣ��Я���ߣ�Ϊ���ᣬ������ɵ�λD�Ǻ�������ͼ���Ƕ��ǵ���ɣ���ѿ������2������������ɵģ����A�������ǣ���������1���������Ǻ�1���ӹ�����ɵģ����B�ǹ��ǣ���������1���������Ǻ�1���Ӱ�������ɣ����C�ǰ�������
��1������ϸ���������Ԫ����CԪ�أ��������ں��������л��������ǵ����ʣ���ͼ�е�G��
��2���ɷ�����֪��ͼ���е�D�Ǻ����ᣬ�������Ԫ����C��H��O��N��P������C�ǰ����ᣬ��ɵ����ʵİ��������R����ͬ��Ϊ20����
��3��С����������Ϊ��Դ���ʵĶ���E��Ҫ�ǵ�����
��4����E������ȣ�F֬���е�H�ĺ����϶࣬�����ֽ�ʱ���ĵ������϶���
��5���ɷ�����֪��B�ǹ��ǣ�C�ǰ����ǣ������Dz���ˮ����ǣ����ۡ���ά�ء���ԭ�ȶ��ǵĵ��嶼����������
��6��������������ѿ��������������ԭ������֪�������ܺϳ���ԭ�����һ���Ƕ�����

����Ŀ��ͼ1��ʾ�ĵ�ѭ������̬ϵͳ����ѭ������Ҫ��ɲ��֣������Ӿ��˵�ѭ���е�����ת����

��1�������ͼ�ж�����˵����ȷ����________������ĸ��ţ���
A. �̵������У�N2ֻ��������
B. ������ϸ�������·���������������Ҫ������������
C. �����������������ֲ��˹��̵��Ե�ѭ����ɵ�Ӱ��
D. ͬ�������������У���Ԫ�ؾ�������ת�����л���
��2�����������У�NH3ת����HNO2�ķ�Ӧ�Ļ�ѧ����ʽΪ_______��
��3�������������У�CH3OH����Ϊ��Ӧ�Ļ�ԭ�����뽫�÷�Ӧ�����ӷ���ʽ����������5CH3OH + 6NO3- ![]() N2�� + 4HCO3- +��______+��
N2�� + 4HCO3- +��______+��
��4�������±����ݽ��й��㣬д����ҵ�ϳɰ���Ӧ���Ȼ�ѧ����ʽ��_______��
���ۼ� | N��N | H��H | N��H |
�Ͽ�1mol���ۼ�����������kJ�� | 946 | 436 | 391 |
��5����ⷨ�ϳɰ�����ԭ��ת���ʴ������ߣ��������洫ͳ�Ĺ�ҵ�ϳɰ����ա���ⷨ�ϳɰ�������ԭ����װ����ͼ2��ͼ3��ʾ��

��ͼ2�У�a�缫��ͨ���XΪ_______��
��ͼ3�У�d�缫�ϵĵ缫��ӦʽΪ_______��
����ͼ2��ͼ3װ�õ�ͨ��ʱ����ͬ������ǿ����ȣ����Ч�ʷֱ�Ϊ80%��60%��������װ���в������������ʵ���֮��Ϊ_______��
����Ŀ����. �ס��ҡ��������ֲ�����ͬ���ӵĿ�����ǿ����ʣ�������������������ʾ��
������ | NH4+��Mg2+��Ba2+ |
������ | OH����NO3����Cl�� |
ȡ�����������ֻ�����������ͬ�������Һ�������ʵ����ʵ���Ũ�ȣ�c(��)>c(��)>c(��)��
��1������_________________
��2��������__________________�����ʵ��ȷ�����������_______________________���������������ȷ������˿ղ��
��. ��ͼΪʵ����ijŨ�����Լ�ƿ�ı�ǩ���Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺

��1����Ũ������HCl�����ʵ���Ũ��Ϊ__________��
��2��ijѧ����������Ũ���������ˮ����500 mL���ʵ���Ũ��Ϊ0.40 mol/L��ϡ���ᡣ
�ٸ�ѧ������Ͳ��ȡ________mL����Ũ����������ơ�
�������ƹ����У�����ʵ��������������������ʵ���Ũ���к�Ӱ�죿���ڿո����� ��ƫ�ߡ�����ƫ�͡�����Ӱ�족����
����ʱ���ӹ۲�_________��
���ݺ���ҡ�ȡ����ú���Һ���½����ټ�����������ˮ__________��
����Ŀ��������������{[CH3CH(OH)COO]2Fe��3H2O}��һ�ֺܺõ�ʳƷ��ǿ����,������ˮ,����Ч���������ã�����������FeCO3��Ӧ�Ƶ�:
2CH3CH(OH)COOH+FeCO3+2H2O��[CH3CH(OH)COO]2Fe��3H2O +CO2����
I.�Ʊ�̼������:װ����ͼ��ʾ��

(1)C��������___________��
(2)��ϴ����,���װ��������,A�м�������,B�м�������,C�м���NH4HCO3��Һ��Ϊ˳�����ʵ��Ŀ��,����װ���л����Ĵر�˳��Ϊ:�رջ���______������______���������������,�رջ���1,��Ӧһ��ʱ���,�رջ���____,����_____��C�з����ķ�Ӧ�����ӷ���ʽΪ_______________��
��.�Ʊ�������������:
���Ƶõ�FeCO3����������Һ��,������������,��75���½���ʹ֮��ַ�Ӧ��Ȼ���ټ����������ᡣ
(3)�����������۵�������______________����������Һ�л�������������������ʵ������Ǹ�����������������________�����
��.�����������崿�ȵIJ���:
(4)����KMnO4�ζ����ⶨ��Ʒ��Fe2+�����������㴿��ʱ,���ֽ�����Ǵ���100%,��ԭ�������_____________________��
(5)����������,����Ce(SO4)2����Һ�ζ����вⶨ����Ӧ��Ce4+���ӵĻ�ԭ����ΪCe3+���ⶨʱ,�ȳ�ȡ5.760g��Ʒ,�ܽ����б�Ҫ������������ƿ���Ƴ�250mL��Һ,ÿ��ȡ25.00mL,��0.1000mol/LCe(SO4)2����Һ�ζ����յ�,��¼�������±���
�ζ����� | 0.1000mol/LCe(SO4)2����Һ���/mL | |
�ζ�ǰ���� | �ζ������ | |
1 | 0.10 | 19.85 |
2 | 0.12 | 21.32 |
3 | 1.05 | 20.70 |
���Ʒ��������������Ĵ���Ϊ_________(������������ʾ)��