��Ŀ����
����Ŀ����Ԫ��������Ŵ����ʺ���Ļ����ɷ�֮һ������Ԫ��(Sn)�γɵ�ijЩ�������ܹ��ٽ�����ĺϳɡ��ش��������⡣
(1)��̬��ԭ�ӵ���ռ�ݵ�����ܲ������____��ռ�ݸ��ܲ�ĵ�����������ߵĵ�����������ڿռ���_____����չ����ԭ�ӹ����_____�Ρ�
(2)��Ԫ�ؿ��γɰ������������������ֵ��ʡ��о���������Ľṹ��õ�������_____��������������ʯ�ṹ��ͬ�����������ȶ�����ԭ����_____��
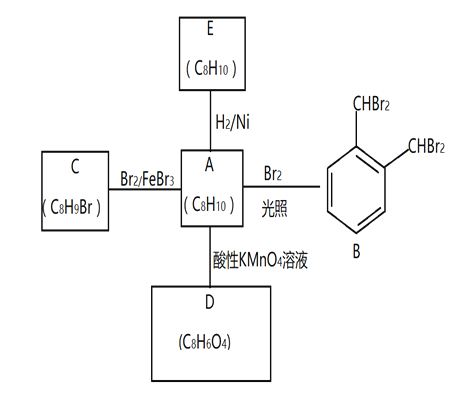
(3)��̬PCl5�ṹ�д���PCl4+��PCl6-���ֽṹ��Ԫ���侧����ͼ��ʾ��

��PCl4+�Ŀռ�ṹΪ________��PCl3�ļ���С��PCl4+���ǵ�ԭ��Ϊ___________��
����֪�����ı߳�Ϊanm,�����ӵ�����ֵ��NA��ʾ����PCl4+��PCl6-֮�����̾���Ϊ_______pm,��̬PCl5���ܶ�Ϊ______g.cm-3��
���𰸡� M 3 ���� X-���������� Sn-Sn���ļ�����������С,�ʻ������ȶ� �������� PCl3��PCl4+����ԭ�ӵ��ӻ���ʽ��Ϊsp3�ӻ�, PCl3��ԭ�Ӽ۲���Ӷ�����һ�Թµ��Ӷ�, PCl4+��ԭ�Ӽ۲���Ӷ����µ��Ӷԣ��µ��ӶԶԳɼ����ӶԵ��ų������ڳɼ����Ӷ�֮����ų��� ![]()
![]()
��������(1)��Ϊ15��Ԫ�أ���̬��ԭ�ӵ���ռ�ݵ�����ܲ������M��ռ�ݸ��ܲ�ĵ�����������ߵĵ���Ϊ3p����������ڿռ���3����չ����ԭ�ӹ���������Σ��ʴ�Ϊ��M��3��������
(2)�о�����Ľṹ��õ�������X-������������������������ʯ�ṹ��ͬ��Sn-Sn���ļ����ϳ������ܽ�С�����»������ȶ����ʴ�Ϊ��X-������������Sn-Sn���ļ�����������С���ʻ������ȶ���
��3����PCl4+������ԭ��P�ļ۲���Ӷ���=4+![]() =4������sp3�ӻ����ռ�ṹΪ����������PCl3��PCl4+����ԭ�ӵ��ӻ���ʽ��Ϊsp3�ӻ�, PCl3��ԭ�Ӽ۲���Ӷ�����һ�Թµ��Ӷ�, PCl4+��ԭ�Ӽ۲���Ӷ����µ��Ӷԣ��µ��ӶԶԳɼ����ӶԵ��ų������ڳɼ����Ӷ�֮����ų�����ʹ��PCl3�ļ���С��PCl4+���ǣ��ʴ�Ϊ������������PCl3��PCl4+����ԭ�ӵ��ӻ���ʽ��Ϊsp3�ӻ�, PCl3��ԭ�Ӽ۲���Ӷ�����һ�Թµ��Ӷ�, PCl4+��ԭ�Ӽ۲���Ӷ����µ��Ӷԣ��µ��ӶԶԳɼ����ӶԵ��ų������ڳɼ����Ӷ�֮����ų�����
=4������sp3�ӻ����ռ�ṹΪ����������PCl3��PCl4+����ԭ�ӵ��ӻ���ʽ��Ϊsp3�ӻ�, PCl3��ԭ�Ӽ۲���Ӷ�����һ�Թµ��Ӷ�, PCl4+��ԭ�Ӽ۲���Ӷ����µ��Ӷԣ��µ��ӶԶԳɼ����ӶԵ��ų������ڳɼ����Ӷ�֮����ų�����ʹ��PCl3�ļ���С��PCl4+���ǣ��ʴ�Ϊ������������PCl3��PCl4+����ԭ�ӵ��ӻ���ʽ��Ϊsp3�ӻ�, PCl3��ԭ�Ӽ۲���Ӷ�����һ�Թµ��Ӷ�, PCl4+��ԭ�Ӽ۲���Ӷ����µ��Ӷԣ��µ��ӶԶԳɼ����ӶԵ��ų������ڳɼ����Ӷ�֮����ų�����
�ڸ��ݾ����ṹ��֪��PCl4+��PCl6-�ĸ�����Ϊ1:1����֪�����ı߳�Ϊanm����PCl4+��PCl6-֮�����̾���Ϊ������Խ��ߵ�һ�룬Ϊ![]() a��103 pm�������к���2��P��10��Clԭ�ӣ���̬PCl5���ܶ�Ϊ
a��103 pm�������к���2��P��10��Clԭ�ӣ���̬PCl5���ܶ�Ϊ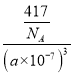 g.cm-3=
g.cm-3=![]() g.cm-3���ʴ�Ϊ��
g.cm-3���ʴ�Ϊ�� ![]() a��103��
a��103�� ![]() ��
��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ����. �ס��ҡ��������ֲ�����ͬ���ӵĿ�����ǿ����ʣ�������������������ʾ��
������ | NH4+��Mg2+��Ba2+ |
������ | OH����NO3����Cl�� |
ȡ�����������ֻ�����������ͬ�������Һ�������ʵ����ʵ���Ũ�ȣ�c(��)>c(��)>c(��)��
��1������_________________
��2��������__________________�����ʵ��ȷ�����������_______________________���������������ȷ������˿ղ��
��. ��ͼΪʵ����ijŨ�����Լ�ƿ�ı�ǩ���Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺
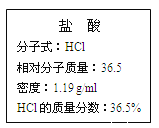
��1����Ũ������HCl�����ʵ���Ũ��Ϊ__________��
��2��ijѧ����������Ũ���������ˮ����500 mL���ʵ���Ũ��Ϊ0.40 mol/L��ϡ���ᡣ
�ٸ�ѧ������Ͳ��ȡ________mL����Ũ����������ơ�
�������ƹ����У�����ʵ��������������������ʵ���Ũ���к�Ӱ�죿���ڿո����� ��ƫ�ߡ�����ƫ�͡�����Ӱ�족����
����ʱ���ӹ۲�_________��
���ݺ���ҡ�ȡ����ú���Һ���½����ټ�����������ˮ__________��