��Ŀ����
11�����ǻ��õļ�������غ����������أ�K2O�����������أ�K2O2���ͳ������أ�KO2���ȶ��ֻ������1���غ�����ط�Ӧ���Ƶ�K2O��10K+2KNO3��6K2O+N2����39.0g����10.1g����س�ַ�Ӧ����K2O������Ϊ28.2g����д����Ҫ�Ĺ��̣�
��2��ij����������Ʒ�������������������ʲ�������Ϊ0.28������Ʒ��K2O2����������Ϊ96.25%����д����Ҫ�Ĺ��̣�
��3���������غͶ�����̼��Ӧ����������4KO2+2CO2��2K2CO3+3O2������ҽԺ����DZˮ���߿շ�����������������13.2L����״����CO2��KO2��Ӧ�����������Ϊ18.8L ����״���������㷴Ӧ���ĵ�KO2����������д����Ҫ�Ĺ��̣�
��4��KO2������600�沿�ַֽ�õ�����A����K2O2��KO2����6.30g����A������ն�����̼����̼��ز���������1.12L����״��������ȷ������A�м�������ԭ�Ӹ���֮�ȣ���д����Ҫ�Ĺ���2K2O2+2CO2��2K2CO3+O2��
���� ��1���ֱ����ء�����ص����ʵ�������Ϸ���ʽ���㣻
��2��K2O2��K2O2������������110��32��
��3��13.2L����״����CO2��KO2��Ӧ�����������Ϊ18.8L���ɽ�Ϸ���ʽ�����ò��������㣻
��4����A��ƽ����ѧʽΪ KxOy�����ݷ�ӦKxOy+$\frac{x}{2}$CO2�T$\frac{x}{2}$K2CO3+��y-0.5x��O2���м��㣮
��� �⣺��1��n��K��=$\frac{39g}{39g/mol}$=1mol��n��KNO3��=$\frac{10.1g}{101g/mol}$=0.1mol���ɷ���ʽ10K+2KNO3��6K2O+N2��֪��K�������������ȫ��Ӧ����Ӧ����0.3molK2O��
������0.3mol��94g/mol=28.2g��
�ʴ�Ϊ��28.2��
��2��K2O2��K2O2������������110��32��ij����������Ʒ�������������������ʲ�������Ϊ0.28������Ʒ��K2O2����������Ϊ0.28��$\frac{110}{32}$��100%=96.25%��
�ʴ�Ϊ��96.25%��
��3��4KO2+2CO2��2K2CO3+3O2 ��V
4mol 4.8L 67.2L 22.4L
n 5.6L
n=$\frac{4mol��5.6L}{22.4L}$=1mol��
m��KO2��=1mol��71g/mol=71g��
�𣺷�Ӧ���ĵ�KO2������Ϊ71g��
��4����A��ƽ����ѧʽΪ KxOy��
KxOy+$\frac{x}{2}$CO2�T$\frac{x}{2}$K2CO3+$\frac{1}{2}$��y-0.5x��O2
39x+16y 11.2��y-0.5x��
6.3 1.12
���x��y=2��3��
����n��K����n��O��=2��3��
����A�м�������ԭ�Ӹ���֮��2��3��
��A�м�������ԭ�Ӹ���֮��Ϊ2��3��
���� ���⿼��������ԭ��Ӧ��Ϊ�߿��������ͣ�����������ԭ��Ӧ�л�������Ŀ��飬��ȷ��Ԫ�ػ��ϼ۽��͵�����Ϊ����������ԭ���������ȼ��ɽ����Ŀ�ϼ�

 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д� �����߿����ϵ�д�
�����߿����ϵ�д�| A�� | 5.6 g Fe ��������HCl��ȫ��Ӧʧȥ������Ϊ0.3 NA | |
| B�� | 22.4L O2����NA��O2���� | |
| C�� | 0.2 mol/L CaCl2��Һ�к���Cl�����ӵ���ĿΪ0.4NA | |
| D�� | 1.6g CH4�����ĵ�����ΪNA |
��1����ˮ����ͨ�����ȵ�̼���ɲ���ˮú����
��ӦΪ��C��s��+H2O��g��?CO��g��+H2��g����H=+131.3kJ•mol-1��
�ٸ÷�Ӧ�ڸ��������Է����У���ܡ����ܡ�����
�ں����£����ݻ�������ܱ������У��������Ͽ��淴Ӧ��һ��ʱ��������������������仯ʱ���ܱ����÷�Ӧ�Ѵﵽƽ��״̬���У�D��
����������ܶȣ��������������ѹǿ����������������ʵ�������CO���ʵ���Ũ��
A��ֻ�Т�B��ֻ�Т�͢�C��ֻ�Т�͢�D����͢�
��2��ˮú���ٽ�һ����Ӧ����ȡ��������ӦΪH2O��g��+CO��g��?H2��g��+CO2��g����ij�¶��¸÷�Ӧ��ƽ�ⳣ��K=4/9�����¶����ڼס��ҡ������������ܱ������У�ֻͶ��H2��g����CO2��g��������ʼŨ�����±���ʾ�������жϲ���ȷ����C��
| ��ʼŨ�� | �� | �� | �� |
| c��H2��/mol/L | 0.010 | 0.020 | 0.020 |
| c��CO2��/mol/L | 0.010 | 0.010 | 0.020 |
B��ƽ��ʱ�����кͱ���H2��ת���ʾ���60%
C��ƽ��ʱ������c��CO2���Ǽ��е�2������0.012mol/L
D��ƽ��ʱ������CO2��ת���ʴ���60%
| A�� | �����£���pH��ֽ���ij������ˮ��pHֵΪ9 | |
| B�� | �ñ�������Һ�ζ�һ������Ĵ���NaOH��Һʱ����ʯ����ָʾ�� | |
| C�� | �ü�ʽ�ζ�����ȡ���������Һ5.10 mL | |
| D�� | ��10 mL����Ͳ��ȡ8.5 mLŨ���� |
| A�� | �������ƺ���ˮ��Ӧ��Na2O2+H2O�T2Na++2OH-+H2�� | |
| B�� | ����Ͷ�뵽NaOH��Һ�У�2Al+2OH-�T2AlO2-+H2�� | |
| C�� | ̼������ڴ��CaCO3+2H+�TCa2++CO2��+H2O | |
| D�� | �Ȼ�������Һ��ͨ��������2Fe2++Cl2�T2Fe3++2Cl- |
 ijͬѧ������װ�ã��̶����������������ԣ������йذ�����ȡ��ʵ��̽�����ش��������⣮
ijͬѧ������װ�ã��̶����������������ԣ������йذ�����ȡ��ʵ��̽�����ش��������⣮
 ��
��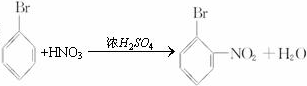 ��
�� ��
��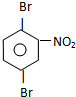 ��
�� ����β����δ��Ӧ�������ͷ�ֹ������ˮ��������װ��D��
����β����δ��Ӧ�������ͷ�ֹ������ˮ��������װ��D��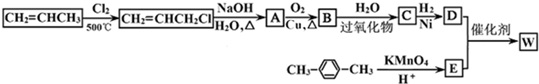
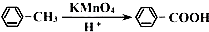
 ��
�� ��
�� ��
�� ��
��