��Ŀ����
19��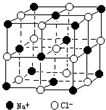 ��1���۵�е�HF��HI��ԭ��HF���Ӽ京�����
��1���۵�е�HF��HI��ԭ��HF���Ӽ京�������2�����ֺ�����HClO��HClO3��H2SO3��HClO4��������ǿ��������ΪHClO4��HClO3��H2SO3��HClO
��3������4�������۵�е��ɸߵ�������Ϊ�٣��ܣ��ۣ��ڣ�����ţ�
�ٽ��ʯ��C-C�� ���ࣨGe-Ge�� �۾���裨Si-Si���ܽ��ɰ��Si-C��
��4��������й���ļ������Σ���������������ظ���Ԫ��֮Ϊ������NaCl����ṹ��ͼ��ʾ��������ÿ��Na+ͬʱ������6��Cl-������Щ Cl-���ɵļ��ι���Ϊ�������壬��������ÿ��Cl-��Χ������ӽ��Ҿ�����ȵ�Cl-����12����
��5��ijԪ�صļ���̬ԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p34s1�����Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p4��������������Ӧ��ˮ����Ļ�ѧʽ��H2SO4��
��6��ijԪ��ԭ�ӵļ۵��ӹ���Ϊ3d104s2�������ڵ������ڣ���IIB�壬
ds��Ԫ�أ�Ԫ�ط�����Zn����ԭ�Ӻ�����7���ܼ��������Ƶ���״��3�֣�
���� ��1���⻯�ﶼ�Ƿ��Ӿ��壬�۷е�������Է������������ȣ�������������۷е�ϸߣ�
��2��Ԫ�صķǽ�����Խǿ��������������ˮ��������Խǿ��ͬһԪ�صIJ�ͬ�����ᣬ���з��ǻ���ԭ�Ӹ���Խ��������Խǿ��
��3��ԭ�Ӿ����۷е�������ɷ��ȣ�
��4��������ÿ��Na+ͬʱ������6��Cl-������ЩCl-���ɵļ��ι���Ϊ�������壬��������ÿ��Cl-��Χ������ӽ��Ҿ�����ȵ�Cl-����=3��8��2��
��5��ijԪ�صļ���̬ԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p34s1����Ԫ��ԭ�Ӻ�����16�����ӣ����ݹ���ԭ����д���̬ԭ�Ӻ�������Ų�ʽ����Ԫ����SԪ�أ�������������ˮ���������
��6�����ݸ�Ԫ�ؼ۵����Ų�ʽ֪����Ԫ��λ�ڵ������ڵ�IIB�壬����ds������ԭ�Ӻ�������ܼ���=1+2+3+1=7����������״�����Ρ��Ĵ��Ρ������Σ�
��� �⣺��1���⻯�ﶼ�Ƿ��Ӿ��壬�۷е�������Է������������ȣ�������������۷е�ϸߣ�HF�к�������������۷е�HF��HI���ʴ�Ϊ������HF���Ӽ��������
��2��Ԫ�صķǽ�����Խǿ��������������ˮ��������Խǿ��ͬһԪ�صIJ�ͬ�����ᣬ���з��ǻ���ԭ�Ӹ���Խ��������Խǿ�����������Դ��ڴ����ᣬ��������ǿ��˳���ǣ��ʴ�Ϊ��HClO4��HClO3��H2SO3��HClO��
��3��ԭ�Ӿ����۷е�������ɷ��ȣ�����Ge-Ge��Si-Si��C-Si��C-C�������۷е�٣��ܣ��ۣ��ڣ�
�ʴ�Ϊ���٣��ܣ��ۣ��ڣ�
��4�����Ȼ��ƾ���������������λ����6������ÿ��Na+ͬʱ������6��Cl-������Щ Cl-���ɵļ��ι���Ϊ�������壬��������ÿ��Cl-��Χ������ӽ��Ҿ�����ȵ�Cl-����=3��8��2=12��
�ʴ�Ϊ��6���������壻12��
��5��ijԪ�صļ���̬ԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p34s1����Ԫ��ԭ�Ӻ�����16�����ӣ����ݹ���ԭ����д���̬ԭ�Ӻ�������Ų�ʽΪ1s22s22p63s23p4����Ԫ����SԪ�أ�������������ˮ���������ᣬ��ѧʽΪH2SO4���ʴ�Ϊ��1s22s22p63s23p4��H2SO4��
��6�����ݸ�Ԫ�ؼ۵����Ų�ʽ֪����Ԫ��λ�ڵ������ڵ�IIB�壬����ds������ԭ�Ӻ�������ܼ���=1+2+3+1=7����������״�����Ρ��Ĵ��Ρ������Σ�
�ʴ�Ϊ���ģ�IIB��ds��Zn��7��3��
���� ���⿼�����ʽṹ�����ʣ�Ϊ��Ƶ���㣬�漰ԭ�Ӻ�������Ų�����λ�����㡢�����֪ʶ�㣬���ؿ���ѧ���ƶϡ����㼰�ռ������������ѵ�����λ�����㣮

| A�� | �� | B�� | ��ϩ | C�� | �Ҵ� | D�� | ���� |
| A�� | ����ױ� | B�� | �Զ��ױ� | C�� | ����ϩ | D�� | ��ϩ |
| A�� | �屽���壩 �����������ٷ�Һ | B�� | ���ᣨ�Ҵ��������� | ||
| C�� | �������������ᣩֱ�ӷ�Һ | D�� | ���飨��ϩ�������Ը��������Һ |
| A�� | ���Ż�ʱ����ĭ��������� | |
| B�� | �ռ���Һ�����ڴ���������ĥ�ڲ����Լ�ƿ�� | |
| C�� | Ũ���ᱣ������ɫϸ���Լ�ƿ�� | |
| D�� | ij��Һ����CCl4��CC14������ɫ��֤��ԭ��Һ�д���I- |
 ����̬ԭ�Ӻ�������Ų�ʽ1s22s22p63s23p63d24s2����Ԫ�ص�ԭ������Ϊ22����Ԫ���ǽ���Ԫ�أ���������ǽ���������
����̬ԭ�Ӻ�������Ų�ʽ1s22s22p63s23p63d24s2����Ԫ�ص�ԭ������Ϊ22����Ԫ���ǽ���Ԫ�أ���������ǽ���������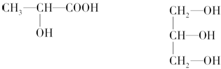
 ��������ͨ����ͬ�ķ�Ӧ�õ��������ʣ�
��������ͨ����ͬ�ķ�Ӧ�õ��������ʣ� C��
C�� D��
D��
 ��
��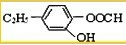 ��
�� ��
��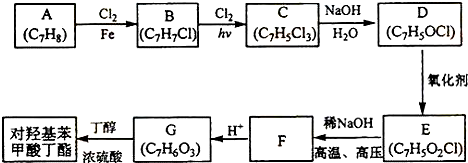
 ���÷�Ӧ������Ϊȡ����Ӧ��
���÷�Ӧ������Ϊȡ����Ӧ�� ��
��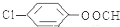 ��д�ṹ��ʽ����
��д�ṹ��ʽ����