��Ŀ����
3����1��������NO2������ܱ���ƿ�����ˮ�У���ƿ��������ɫ��dz�������������䡱��dz����������ƽ���ƶ�ԭ��˵���仯ԭ��2NO2?N2O4��H��0�������¶ȣ�ƽ������ȷ�������Ӧ�����ƶ������¶������������Ũ�Ƚ��ͣ���2����80��ʱ����0.40mol N2O4�������2L�Ѿ���յĹ̶��ݻ����ܱ������з�����Ӧ����һ��ʱ��Ը������ڵ����ʽ��з������õ�������ݣ�
| ʱ�䣨s�� | 0 | 20 | 40 | 60 | 80 | 100 |
| n��N2O4��/mol | 0.40 | a | 0.20 | c | d | e |
| n��NO2��/mol | 0.00 | 0.24 | b | 0.52 | 0.60 | 0.60 |
�ڸı�����ʹ��Ӧ���´ﵽƽ�⣬��ʹ$\frac{c��N{O}_{2}��}{c��{N}_{2}{O}_{4}��}$ֵ��С�Ĵ�ʩ�У�����ţ�A��
A������N2O4����ʼŨ�� B�������¶� C��ʹ�ø�Ч���� D������������ͨ��ϡ�����壮
���� ��1����������������������֮�����ƽ��ת��2NO2?N2O4�Ƿ��ȷ�Ӧ�������¶ȣ�ƽ������ȷ����ƶ������ݶ�����������Ũ�ȱ仯�ж�������ɫ�仯��
��2��������0.24mol����������Ҫ0.12mol�����������ֽ⣻��K=$\frac{������Ũ��ϵ���ݴη�}{��Ӧ��Ũ��ϵ���ݴη�}$���㣻
����ʹ$\frac{c��N{O}_{2}��}{c��{N}_{2}{O}_{4}��}$ֵ��С����ʹƽ�����淴Ӧ�����ƶ����ɣ�
��� �⣺��1��2NO2?N2O4 ��H��0���Ƿ��ȷ�Ӧ�������¶ȣ�ƽ������ȷ�������Ӧ�����ƶ������¶������������Ũ�Ƚ��ͣ��������ɫ��dz��
�ʴ�Ϊ����dz��2NO2?N2O4��H��0�������¶ȣ�ƽ������ȷ�������Ӧ�����ƶ������¶������������Ũ�Ƚ��ͣ�
��2��������0.24mol����������Ҫ0.12mol�����������ֽ⣬a=0.40mol-0.12mol=0.28mol��
��ϻ�ѧƽ������ʽ��ʽ����
N2O4��g���T2NO2��g��
��ʼ����mol�� 0.4 0
�仯����mol�� 0.3 0.6
ƽ������mol�� 0.1 0.6
K=$\frac{������Ũ��ϵ���ݴη�}{��Ӧ��Ũ��ϵ���ݴη�}$=$\frac{��\frac{0.6mol}{2L}��^{2}}{\frac{0.1mol}{2L}}$=1.8��
�ʴ�Ϊ��0.28mol��1.8��
����ʹ$\frac{c��N{O}_{2}��}{c��{N}_{2}{O}_{4}��}$ֵ��С����ʹƽ�����淴Ӧ�����ƶ���
A������N2O4����ʼŨ�ȣ�����������ԭ����ƽ���ƶ��Ľ��ֻ�ܼ������ָı䣬�������������ԣ�c��NO2��/c��N2O4��ֵ��С����A��ȷ��
B�������¶Ȼ�ʹƽ�������ɶ��������ķ����ƶ�����ʹc��NO2��/c��N2O4��ֵ���B����
C������ֻ�ܼӿ컯ѧ��Ӧ���ʣ�����Ӱ��ƽ���ƶ�����C����
D������������ͨ��ϡ�����壬��Ӧ���������Ũ�Ȳ��䣬��Ӱ��ƽ���ƶ���c��NO2��/c��N2O4��ֵ���䣬��D����
�ʴ�Ϊ��A��
���� ���⿼����ƽ�ⳣ���ļ����ƽ���ƶ����жϣ���Ŀ�ѶȲ���

| A�� | B��C��D�γɵļ����ӣ��뾶��С����B | |
| B�� | E�γ�����⻯���ȶ��Ա�Bǿ | |
| C�� | A��C��D�γɵ�����������ˮ��������������Ӧ | |
| D�� | A��B�γɵĻ�������B��E�γɵĻ����ﻯѧ��������ͬ |
| A�� | ����50% | B�� | ����50% | C�� | С��50% | D�� | ��ȷ�� |
| A�� | ��ͭпԭ�����CuΪ���� | |
| B�� | ԭ����У���ѧ��ת��Ϊ���� | |
| C�� | ��ԭ����У������Ϸ���������Ӧ�������Ϸ�����ԭ��Ӧ | |
| D�� | ��ԭ����У����ӴӸ��������������Һ�������� |
 ��ͼװ�ÿ���������ȡijЩ���壮
��ͼװ�ÿ���������ȡijЩ���壮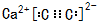 �������ʽ����Һ©����ʢ�ŵ��DZ���ʳ��ˮ��Ŀ����ʹ��Ӧ������Ȳ������ƽ����
�������ʽ����Һ©����ʢ�ŵ��DZ���ʳ��ˮ��Ŀ����ʹ��Ӧ������Ȳ������ƽ����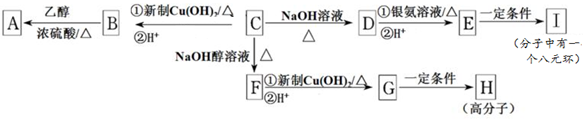
 ��I�Ľṹ��ʽ��
��I�Ľṹ��ʽ��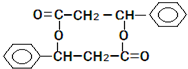 ��
�� ��
�� ��
�� ��
��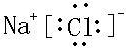 ��
�� ��
��