��Ŀ����
11����ͼ��ʾ����һ�����ʵ���Ũ����Һ�ļ����ؼ�ʵ�鲽��Ͳ�������������230mL 0.100mol•L-1 Na2CO3��Һ���ش��������⣺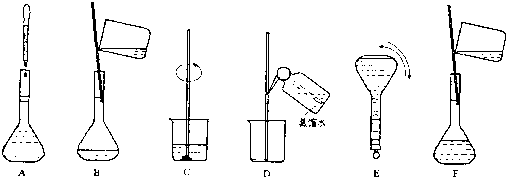
��1������Na2CO3��10H2O��������Һ����ѡ��250mL������ƿ������ƽ��ȡ����7.2�ˣ�����ȡ�ľ����Ѿ���һ����ʧȥ�˽ᾧˮ���������Ƶ���ҺŨ��ƫ����ƫ��ƫС����Ӱ�죩��
��2������Bͨ����Ϊת�ƣ�������������������������Aͨ����Ϊ���ݣ���ʱӦע�������밼Һ�桢�̶���ˮƽ���У�������ӿ̶��ߣ����Ƶ�Ũ��ƫ�� ����ƫ��ƫС����Ӱ�죩�������������Һ����ڿ̶��ߣ������Ƶ�Ũ����Ӱ�죨��ƫ��ƫС����Ӱ�죩��
��3��������ʵ�鲽��A-F��ʵ������Ⱥ��������C��B��D��F��A��E��
��4�����й�������ƿʹ�õ�˵����ȷ����BD
A��ʹ��ǰ�����
B��ʹ��ǰ�ȼ��ƿ����©ˮ
C����õ���Һ��������������ƿ��
D������Һ����ȴ�����²���ת��������ƿ
E������ƿ����ȷ�������������һ�����ʵ���Ũ�ȵ���Һ��
���� ��1��������230mL 0.100mol•L-1 Na2CO3��Һ����Ҫѡ��250mL����ƿ��ʵ������250mL��Һ������m=CVM�������ʵ�����������ȡ�ľ����Ѿ���һ����ʧȥ�˽ᾧˮ�����³�ȡ�����ʵ����ʵ���ƫ������C=$\frac{n}{V}$�ж���
��2����������һ�����ʵ���Ũ����Һ����Һ�����ݵ���ȷ�������������������ʵ����ʵ�������Һ�����Ӱ�죬����C=$\frac{n}{V}$�ж����ݴ˽��
��3����������һ�����ʵ���Ũ����Һһ�㲽������
��4������ƿΪ����һ�����ʵ���Ũ�ȵ���Һ�ļ���������ֻ���ڳ�����ʹ�ã���ͬ�Ĺ�������ƿ���Ƶ���Һ�������ͬ������ʹ��ǰӦ����Ƿ�©Һ��
��� �⣺��1��������230mL 0.100mol•L-1 Na2CO3��Һ����Ҫѡ��250mL����ƿ��ʵ������250mL��Һ����ҪNa2CO3��10H2O������m=CVM=0.100mol/L��0.25L
��286g/mol=7.2g������ȡ�ľ����Ѿ���һ����ʧȥ�˽ᾧˮ�����³�ȡ�����ʵ����ʵ���ƫ������C=$\frac{n}{V}$��֪����ҺŨ��ƫ��
�ʴ�Ϊ��250mL��7.2�� ƫ��
��2������һ�����ʵ���Ũ����Һ����Һ�����в�����������������������Aͨ����Ϊ���ݣ���ʱӦע�������밼Һ�棬�̶���ˮƽ���У�������ӿ̶��ߣ�������Һ���ƫС����ҺŨ��ƫ�����������Һ����ڿ̶��ߣ��������������������ʵ����ʵ�������Һ�����������Ӱ����ҺŨ�Ȳ��䣻
�ʴ�Ϊ�����������ݣ���Һ�棬�̶��ߣ�ƫ����Ӱ�죻
��3������һ�����ʵ���Ũ����Һһ�㲽�裺���㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȣ�������ȷ��˳��Ϊ��C��B��D��F��A��E��
�ʴ�Ϊ��C��B��D��F��A��E��
��4��A������ƿ������Һʱ����Ҫ����������Ҫ��������ˮ��ʹ��ǰ��������A����
B������ƿʹ��ǰ�ȼ��ƿ����©ˮ����B��ȷ��
C������ƿ�����������ڴ洢��Һ����C����
D������ƿΪ����������ֻ��������������ž�ȷ����D��ȷ��
E������ƿ����ȷ����һ�������һ�����ʵ���Ũ�ȵ���Һ�������������������Һ����E����
��ѡ��BD��
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƣ���ȷ����ԭ���Ͳ����ǽ���ؼ���ע������ƿ��ѡ���ʹ�÷�������Ŀ�ѶȲ���

 ���ٴ�����ɽ����ϵ�д�
���ٴ�����ɽ����ϵ�д�| A�� | �ڳ��³�ѹ�£�11.2L N2���еķ�����ΪNA | |
| B�� | ��״���£�18g H2O��ռ�����Լ��22.4L | |
| C�� | 32gO2�ڱ�״������ռ�����ԼΪ22.4L | |
| D�� | 1mol Ne��Լ����6.02��1024������ |
| A�� | N4��N2�ǻ�Ϊͬλ�� | |
| B�� | N4��Ħ��������56g | |
| C�� | ��ͬ������N4��N2����ԭ�Ӹ�����Ϊ2��1 | |
| D�� | ÿ��N4���Ӻ���28������ |
| ���� | ���� | ���� | |
| A | �μ�ϡ���� | �����ݲ��� | ԭ��Һ����CO32- |
| B | �μ������ữ�� BaCl2��Һ | ���ɰ�ɫ���� | ԭ��Һ����Ag+ ��SO42- |
| C | �ýྻ��˿պȡ��Һ �������� | ����ʻ�ɫ | ԭ��Һ����Na+�� ��K+ |
| D | �μ�ϡNaOH��Һ����ʪ ���ɫʯ����ֽ�����Թܿ� | ��ֽ������ | ԭ��Һ����NH4+ |
| A�� | A | B�� | B | C�� | C | D�� | D |
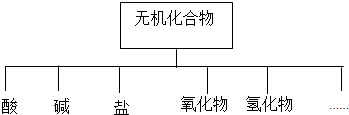
��1����ͼ��ʾ�����ʷ������������״���෨��
��2����K��Na��H��O��S��N�������ֻ�����Ԫ����ɺ��ʵ����ʣ��ֱ������±��Тڡ��ܡ��ޡ�����森
| ������� | �� | �� | �� | ������ | �⻯�� |
| ��ѧʽ | ��H2SO4 �� | ��NaOH �� | ��Na2SO4 �� | ��SO2 ��SO3 | ��NH3 �� |
����ˮ�е��뷽��ʽΪ��Na2SO4=2Na++SO42-
��4�����������Т�����������Һ��Ӧ�����ӷ�Ӧ����ʽΪ��SO2+2OH-�TH2O+SO32-��
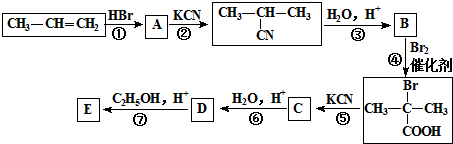
 ��
��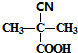
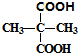 +2C2H5OH$��_{��}^{Ũ����}$
+2C2H5OH$��_{��}^{Ũ����}$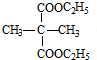 +2H2O��
+2H2O�� ������NaOH��Һ��Ӧ�Ļ�ѧ����ʽΪ
������NaOH��Һ��Ӧ�Ļ�ѧ����ʽΪ ��
��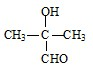 ��
��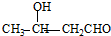 ��
��