��Ŀ����
����Ŀ��ij��ҵ�������к���Cr(OH)3��Al2O3��CuO��NiO�����ʣ���ҵ��ͨ���������̻����������õĽ�������ȡNa2Cr2O7��
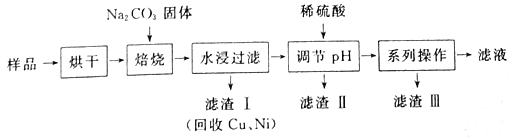
��֪��
����ˮ�������������Һ�д���Na2CrO4��NaAlO2������
�ڳ�ȥ����II����Һ�д��ڷ�Ӧ2CrO42-+2H+![]() Cr2O72-+H2O
Cr2O72-+H2O
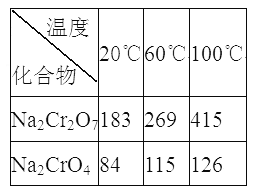
��Na2Cr2O7��Na2CrO4�ڲ�ͬ�¶��µ��ܽ�ȣ�g/100 g H2O)���±���
(1) ������������������NaAlO2��ѧ����ʽΪ_____________________��
(2)��������IIʱ,���������ϡ���������Һ��pH��ȥA1O2-����ϡ��������������������Ӧ�����ӷ���ʽΪ______________________��
(3) ��ϵ�в�����Ϊ����������ϡ���ᡢ_____����ȴ�ᾧ�����ˡ���������ϡ�����Ŀ����_____________������III����Ҫ�ɷ���_________(�ѧʽ)��
(4)��ҵ�ϻ���������ˮ���������������Һ�м�������ϡ���ᣬ��ʯī���缫��������������������ĵ缫��ӦʽΪ______________��
(5)����ͼ���ġ���Һ������Ȼ�в�����Na2Cr2O7��CrΪ�ؽ���Ԫ�أ����������߾���������У���Ժ�ˮ����ؽ�����Ⱦ��Ϊ�ⶨ����Һ���е�c(Na2Cr2O7)��ijʵ��С��ȡ����Һ��20mL��ˮϡ����250 mL����ȡϡ�ͺ����Һ25 mL����ƿ�У���c mol L-1��FeSO4��Һ����������ԭ�ζ������յ�ʱ����FeSO4��Һ���ΪV mL������Һ���е�c(Na2Cr2O7)=______mol��L-1��
���𰸡� A12O3+NaCO3![]() 2NaAlO2+CO2�� A1(OH)3+3H+==Al3++3H2O ����Ũ�� �ٽ���ѧƽ��2CrO42-+2H+
2NaAlO2+CO2�� A1(OH)3+3H+==Al3++3H2O ����Ũ�� �ٽ���ѧƽ��2CrO42-+2H+![]() Cr2O72-+H2O������Ӧ�����ƶ������������ɸ����Na2Cr2O7 Na2Cr2O7 Cr2O72-+14H++12e-==2Cr��+7H2O
Cr2O72-+H2O������Ӧ�����ƶ������������ɸ����Na2Cr2O7 Na2Cr2O7 Cr2O72-+14H++12e-==2Cr��+7H2O ![]()
���������Ժ���Cr(OH)3��Al2O3��ZnO��CuO��NiO�����ʵĵ������Ϊԭ�ϣ���ɺ����̼���ƺ������������շ�����Ӧ4Cr(OH)3+4Na2CO3+3O2![]() 4Na2CrO4+6H2O+4CO2��Al2O3+Na2CO3
4Na2CrO4+6H2O+4CO2��Al2O3+Na2CO3![]() Na2AlO3+CO2����ZnO++Na2CO3
Na2AlO3+CO2����ZnO++Na2CO3![]() Na2ZnO2+CO2����ˮ������˵õ�����CuO��NiO����ҺΪNa2CrO4��NaAlO2��Na2ZnO2�ȣ�������ҺpH����ZnO22-���Ӻ�ƫ��������ӣ����˵õ���ҺNa2CrO4�����������ữ�����ظ�������Һ��ͨ���ᴿ�õ��ظ����ơ�
Na2ZnO2+CO2����ˮ������˵õ�����CuO��NiO����ҺΪNa2CrO4��NaAlO2��Na2ZnO2�ȣ�������ҺpH����ZnO22-���Ӻ�ƫ��������ӣ����˵õ���ҺNa2CrO4�����������ữ�����ظ�������Һ��ͨ���ᴿ�õ��ظ����ơ�
(1) ������������������NaAlO2��ѧ����ʽΪAl2O3+Na2CO3![]() Na2AlO3+CO2�����ʴ�Ϊ��Al2O3+Na2CO3
Na2AlO3+CO2�����ʴ�Ϊ��Al2O3+Na2CO3![]() Na2AlO3+CO2����
Na2AlO3+CO2����
(2)���������������ԣ�������ϡ�����ܹ��ܽ����ɵ�����������������Ӧ�����ӷ���ʽΪA1(OH)3+3H+==Al3++3H2O���ʴ�Ϊ��A1(OH)3+3H+==Al3++3H2O��
(3)ˮ������Һ�д���Na2CrO4���������ᷢ��CrO42-+2H+Cr2O72-+H2O�����������ʹƽ��������Ӧ�����ƶ�������Һ����Ũ������ȴ�ᾧ�ɵõ������Na2Cr2O7���壬�ʴ�Ϊ������Ũ�����ٽ�ƽ��CrO42-+2H+Cr2O72-+H2O������Ӧ�����ƶ������������ɸ����Na2Cr2O7���壻Na2Cr2O7��
(4)���ɸ��ĵ缫������ԭ��Ӧ��Cr2O72-�õ���������Cr����Ӧ�ĵ�ⷽ��ʽΪCr2O72-+14H++12e-==2Cr��+7H2O���ʴ�Ϊ��Cr2O72-+14H++12e-==2Cr��+7H2O��
(5) V mL c mol L-1��FeSO4��FeSO4�����ʵ���ΪcV��10-3mol����Na2Cr2O7��Ӧ���������ӣ�ת�Ƶ���cV��10-3mol�����ݵ�ʧ�����غ㣬Na2Cr2O7ת��ΪCr3+ʱ�õ�cV��10-3mol���ӣ���n(Na2Cr2O7)= ![]() mol����c(Na2Cr2O7)=
mol����c(Na2Cr2O7)=  ��
��![]() =
=![]() mol/L���ʴ�Ϊ��
mol/L���ʴ�Ϊ�� ![]() ��
��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ�������±��ṩ�IJ����������Dz���������ѡ����ʵ��װ�þ��ܴﵽ��Ӧʵ��Ŀ�ĵ���
ѡ�� | A | B | C | D |
ʵ��Ŀ�� | ��ȥKCl������MnO2 | ��10 mol��L-1��������100mL0.1 mol��L-1���� | �ú�������NH4Cl���ʵ�NaCl��Һ�Ʊ��Ȼ��ƾ��� | ����Ȳ�ķ���װ�� |
ʵ��������װ�� | �ձ�������������Һ©�� | 100 mL����ƿ�����������ձ� |
|
|
A. A B. B C. C D. D