��Ŀ����
11�� ����һ�ֿ����ھ�ˮ����ʳƷ���Σ���A��B��C��D��E���ֶ�����Ԫ����ɣ�������ˮ��ɵ�����������ӣ�����һ������A��B�γɵ�10���������ӣ�AԪ��ԭ�Ӻ�����������E����l��D��Eͬ���壮ijͬѧΪ̽������ɶ���������ʵ�飺
����һ�ֿ����ھ�ˮ����ʳƷ���Σ���A��B��C��D��E���ֶ�����Ԫ����ɣ�������ˮ��ɵ�����������ӣ�����һ������A��B�γɵ�10���������ӣ�AԪ��ԭ�Ӻ�����������E����l��D��Eͬ���壮ijͬѧΪ̽������ɶ���������ʵ�飺��ȡmg�ľ�����������ˮ�����500mL��Һ��
��ȡ��������Һ���Թ��У���ε���Ba��OH��2��Һ�����ɳ��������ʵ��������Ba��OH��2��Һ����Ĺ�ϵ��ͼ��ʾ��
��ȡ20mL����Һ���Թ��У��������NaOH��Һ����Ȳ��ռ����������壬Ȼ������ɱ�״���µ����Ϊ224mL��
�ش��������⣺
��1��D��Ԫ�����ڱ��е�λ��Ϊ��������VIA�壮
��2�����ⶨ�����Ħ������Ϊ453g��mol-1�����������Ӻ����������ʵ���֮��Ϊ1��1����1mol�����к���12mol�ᾧˮ������Ļ�ѧʽΪNH4Al��SO4��2•12H2O��
��3��ͼ����V��Oa����V��ab����V��bc��=3��1��1
��4��д��ab�η�����Ӧ�����ӷ���ʽ��2NH4++SO42-+Ba2++2OH-=BaSO4��+2NH3��H2O��
��5����ɵļ���Һ���ʵ���Ũ����0.5mol/L��
���� ����A��B��C��D��E���ֶ�����Ԫ����ɵ�һ���Σ���ȡ��������Һ���Թ��У���ε���Ba��OH��2��Һ�����ɳ�����ʼ���������С�����������ղ���ȫ��ʧ�������Һ�϶�����SO42-��Al3+����ȡ20mL����Һ���Թ��У��������NaOH��Һ����Ȳ��ռ����������壬�����Һ�к���NH4+�������ڵ��봦���������ӣ�A��B�γɵ�10����������ΪNH4+��D��Eͬ���壬����Ӧ�γ�SO42-����AԪ��ԭ�Ӻ�����������E����l����AΪNԪ�ء�EΪOԪ�ء�DΪSԪ�ء�BΪHԪ�ء�CΪAl����2���о��ⶨ�����Ħ������Ϊ453g��mol-1����1mol�����к���12mol�ᾧˮ�����������Ӻ������ӵķ�����Ϊ��453-216=237�������Ӻ����������ʵ���֮��1��1�����ݵ�����ԭ�����仯ѧʽΪ��NH4Al��SO4��2•12H2O��oa�η�����Ӧ��2NH4Al��SO4��2+3Ba��OH��2=3BaSO4��+2Al��OH��3��+��NH4��2SO4��ab�η�����Ӧ����NH4��2SO4+Ba��OH��2=BaSO4��+2NH3��H2O��bc�η�����Ӧ��OH-+Al��OH��3=AlO2-+2H2O���ݴ˽��
��� �⣺����A��B��C��D��E���ֶ�����Ԫ����ɵ�һ���Σ���ȡ��������Һ���Թ��У���ε���Ba��OH��2��Һ�����ɳ�����ʼ���������С�����������ղ���ȫ��ʧ�������Һ�϶�����SO42-��Al3+����ȡ20mL����Һ���Թ��У��������NaOH��Һ����Ȳ��ռ����������壬�����Һ�к���NH4+�������ڵ��봦���������ӣ�A��B�γɵ�10����������ΪNH4+��D��Eͬ���壬����Ӧ�γ�SO42-����AԪ��ԭ�Ӻ�����������E����l����AΪNԪ�ء�EΪOԪ�ء�DΪSԪ�ء�BΪHԪ�ء�CΪAl����2���о��ⶨ�����Ħ������Ϊ453g��mol-1����1mol�����к���12mol�ᾧˮ�����������Ӻ������ӵķ�����Ϊ��453-216=237�������Ӻ����������ʵ���֮��1��1�����ݵ�����ԭ�����仯ѧʽΪ��NH4Al��SO4��2•12H2O��oa�η�����Ӧ��2NH4Al��SO4��2+3Ba��OH��2=3BaSO4��+2Al��OH��3��+��NH4��2SO4��ab�η�����Ӧ����NH4��2SO4+Ba��OH��2=BaSO4��+2NH3��H2O��bc�η�����Ӧ��OH-+Al��OH��3=AlO2-+2H2O��
��1��DΪSԪ�أ��������ڱ��е�������VIA�壬�ʴ�Ϊ����������VIA�壻
��2��������������֪���Ļ�ѧʽΪ��NH4Al��SO4��2•12H2O���ʴ�Ϊ��NH4Al��SO4��2•12H2O��
��3������NH4Al��SO4��2•12H2OΪ2mol��oa�η�����Ӧ��2NH4Al��SO4��2+3Ba��OH��2=3BaSO4��+2Al��OH��3��+��NH4��2SO4������3molBa��OH��2������1mol��NH4��2SO4������2molAl��OH��3��ab�η�����Ӧ����NH4��2SO4+Ba��OH��2=BaSO4��+2NH3��H2O��1mol��NH4��2SO4����1molBa��OH��2��bc�η�����Ӧ��OH-+Al��OH��3=AlO2-+2H2O��2molAl��OH��3����1molBa��OH��2����ͼ����V��Oa����V��ab����V��bc��=3mol��1mol��1mol=3��1��1��
�ʴ�Ϊ��3��1��1��
��4��ab�η�����Ӧ����NH4��2SO4+Ba��OH��2=BaSO4��+2NH3��H2O�����ӷ���ʽΪ��2NH4++SO42-+Ba2++2OH-=BaSO4��+2NH3��H2O��
�ʴ�Ϊ��2NH4++SO42-+Ba2++2OH-=BaSO4��+2NH3��H2O��
��5��ʵ�����ȡ20mL����Һ���Թ��У��������NaOH��Һ����Ȳ��ռ������İ���Ϊ$\frac{0.224L}{22.4L/mol}$=0.01mol����NH4Al��SO4��2Ϊ0.01mol������ҺŨ��Ϊ$\frac{0.01mol}{0.02L}$=0.5mol/L��
�ʴ�Ϊ��0.5mol/L��
���� ���⿼��������ƶϣ��ؼ��Ǹ���ʵ�������ƶϺ��е�������ȷ���η����ķ�Ӧ���Ƕ�ѧ���ۺ������Ŀ��飬�ѶȽϴ�

| A�� | ȼ�ճס������� | B�� | Բ����ƿ������ | C�� | ��Ͳ������ƿ | D�� | �Թܡ���ƿ |
| A�� | ����ͭ����������ȼ�գ�˵��������ͭ����ͨͭ�Ļ�ԭ��ǿ | |
| B�� | ��ɫ��Ⱦ�����������й� | |
| C�� | �����ն����γ��뻯ʯȼ�ϴ���ʹ���й� | |
| D�� | ����ˮ���������Խ������ˮ��ӦΣ��������ˮ�м��뾻ˮ����������ʹ��ˮ���� |
| A�� | �����к���̼������ʴ�����ȴ����� | |
| B�� | ������Ʒ�϶�ͭʱ��ʯīΪ����������Ʒ��������ͭ��Ϊ���Һ | |
| C�� | �������ӵ��������������Ӵ������� | |
| D�� | ȼ�����ϵij���֧���������⣬��Ҫ�����ڸ���������������ѧ��ʴ�� |
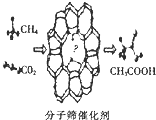 �ҹ���ѧ�ҳɹ��з��˼���Ͷ�����̼�Ĺ�ת�����������÷���ɸ������Ч�Ƶ����ᣬ�����й�˵����ȷ���ǣ�������
�ҹ���ѧ�ҳɹ��з��˼���Ͷ�����̼�Ĺ�ת�����������÷���ɸ������Ч�Ƶ����ᣬ�����й�˵����ȷ���ǣ�������| A�� | ����22.4 LCO2���Ƶ�1mol���� | |
| B�� | �÷�Ӧ����ȡ����Ӧ | |
| C�� | ��Ӧ����������о����м��Լ��ͷǼ��Լ� | |
| D�� | �÷�Ӧ���̷��ϡ���ɫ��ѧ��ԭ������ԭ��������Ϊ100% |
| A�� | NO2��H2O��Ӧ������2mol HNO3ʱת�Ƶĵ�����ĿΪ2NA | |
| B�� | 1mol�������к���3NA��̼̼���� | |
| C�� | 24g NaH��������������������ΪNA | |
| D�� | ��0.1mol/LK2CO3��Һ�У���������Ŀ����0.1NA |
| A�� | ���ۺ���ά�صĻ�ѧʽ���ǣ�C6H10O5��n���ʻ�Ϊͬ���칹�� | |
| B�� | �������ʵ���Ȼ��֬���ڸ߷��ӻ�����й̶����ۡ��е� | |
| C�� | ���ǡ�ֲ���͡���Ȼ��������ȫȼ�պ�����ղ����ΪC02��H20 | |
| D�� | ��ά������������֬�������ʺ���ѿ����һ�������¶���ˮ�� |