��Ŀ����
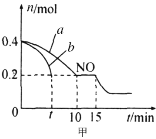
����Ŀ�������£���10.00mL0.1000mol��L-1HCl��0.1000mol��L-1CH3COOH�Ļ����Һ�е���0.1000mol��L-1NaOH��Һ����ҺpH�ı仯������ͼ��ʾ����֪�������£�Ka(CH3COOH)=1.75��10-5�����������������
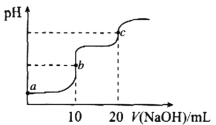
A.a����ʾ��Һ�У�CH3COOH�ĵ����ԼΪ1.75��10-2%
B.a��b��c������ʾ��Һ�У�ˮ�ĵ���̶�������c��
C.c����ʾ��Һ�У�c(Na+)>c(C1-)>c(CH3COOH>c(OH-)>c(H+)
D.����b����ʾ��Һ��![]() ��ֵ��С
��ֵ��С
���𰸡�C
��������
�������Ϣ��֪����10.00mL0.1000mol��L-1HCl��0.1000mol��L-1CH3COOH�Ļ����Һ�е���10mL0.1000mol��L-1NaOH��Һ������պ���ȫ��Ӧ����ʱ��ҺΪNaCl��CH3COOH�Ļ����Һ������20mL0.1000mol��L-1NaOH��Һ����Һ�е�����Ϊ��NaCl��CH3COONa���Ҷ��ߵ����ʵ���Ũ����ȡ��ݴ˽��з�����
A��a����Һ������Դ���ĵ������������ã���ʱc(CH3COOH)Լ����c(H+)������ݵ���ƽ�ⳣ��![]() ���ɵ�
���ɵ�![]() �����ѵ����
�����ѵ����![]() �������ĵ����=
�������ĵ����=![]() ��A����ȷ��
��A����ȷ��
B��a����ҺΪHCl��CH3COOH�Ļ����Һ��b��ҺΪNaCl��CH3COOH�Ļ����Һ��c����Һ�е�����Ϊ��NaCl��CH3COONa����a��b��ˮ�ĵ��붼�ܵ����ƣ�ֻ��c�㣬NaCl��ˮ�ĵ�����Ӱ�죬CH3COO-����ˮ�⣬�ٽ���ˮ�ĵ��룬��a��b��c������ʾ��Һ�У�ˮ�ĵ���̶�������c�㣬B����ȷ��
C��c����ʾ��Һ������Ϊ���ʵ���֮��Ϊ1:1��NaCl��CH3COONa��CH3COO-��������ˮ�⣬������Ũ�ȴ�С��ϵӦΪ��c(Na+)>c(C1-)>c(CH3COO-)>c(OH-)> c(CH3COOH)��c(H+)��C�����
D��b��ʱ����ҺΪNaCl��CH3COOH�Ļ����Һ����ΪCH3COOHΪ������ʣ����Ȼ�ٽ�CH3COOH�ĵ��룬��c(CH3COO-)Ũ������c(Cl-)Ũ���ޱ仯����![]() ��ֵ��С��D����ȷ��
��ֵ��С��D����ȷ��
��ѡC��