��Ŀ����
����Ŀ����(23V)���ҹ��ķ��Ԫ�أ��㷺Ӧ���ڴ���������ҵ��������ѧ�������������ά������֮�ơ��ش��������⣺
(1)��ԭ�ӵĺ�������Ų�ʽΪ________________����Ԫ�����ڱ��е�λ��Ϊ______��
(2)V2O5������SO2ת��ΪSO3�Ĵ�������̬Sԭ�ӵ���ռ������ܼ��ĵ���������Ϊ________�Σ���̬SO3�Ե�������ʽ���ڣ�����ӵ����幹��Ϊ_______�Σ�����SO3�������廷״�ṹ��ͼ��ʾ���ýṹ��S��O������a��b���࣬b�ļ�������a�ļ�����ԭ��Ϊ______________��

(3)V2O5�ܽ���NaOH��Һ�У��ɵõ�������(Na3VO4)��������������V���ӻ��������Ϊ___________��Ҳ�ɵõ�ƫ�����ƣ��������ӳ���ͼ��ʾ��������״�ṹ����ƫ�����ƵĻ�ѧʽΪ_____________��

(4)����ij�������ᄃ���ṹ��ͼ��ʾ����������Ļ�ѧʽΪ__________�������ľ�������Ϊx nm�������ܶ�Ϊ__________ g/cm3��

���𰸡�1s22s22p63s23p63d34s2 ��������VB�� ���� ƽ���������� a������˫���ijɷ֣����ܴ����϶̣�b��Ϊ���������ܽ�С�������ϳ� sp3 NaVO3 VO2 ![]()
��������
(1)V��23��Ԫ�أ�������֪����Ԫ�����ڱ��е�λ��Ϊ��������VB�壻���ݹ���ԭ��֪��1s��2s��2p��3s��3p��4s��3d��4p������ƶ�������Ų�ʽΪ1s22s22p63s23p63d34s2��
(2)S��16��Ԫ�أ����̬ԭ�Ӻ�������Ų�ʽΪ1s22s22p63s23p4��������ܼ���3p�ܼ������������״�������Σ�
SO3��Sԭ�Ӽ۲���Ӷ���=3+![]() =3���Ҳ����µ��Ӷԣ����ݼ۲���ӶԻ������ۣ��ж���ռ乹��Ϊƽ���������Σ�
=3���Ҳ����µ��Ӷԣ����ݼ۲���ӶԻ������ۣ��ж���ռ乹��Ϊƽ���������Σ�
����SO3�������廷״�ṹ��֪���ýṹ����ԭ���γ�4�������ýṹ��S-O ���������࣬һ����ͼ1��a��ʾ��a������˫���ijɷ֣����ܴ����϶�����һ��Ϊb����b��Ϊ���������ܽ�С�������ϳ�������b�ļ�������a�ļ�����
(3)VO43-�У�V�γ�4���������µ��Ӷ���Ϊ![]() ��V�ļ۲���Ӷ���Ϊ4��VΪsp3�ӻ�������״�ṹ��֪ÿ��V��3��O�γ������ӣ���V�Ļ��ϼ�Ϊ+5�ۣ����γɵĻ����ﻯѧʽΪNaVO3��
��V�ļ۲���Ӷ���Ϊ4��VΪsp3�ӻ�������״�ṹ��֪ÿ��V��3��O�γ������ӣ���V�Ļ��ϼ�Ϊ+5�ۣ����γɵĻ����ﻯѧʽΪNaVO3��
(4)�ɾ�����֪��Vԭ��λ�ڶ�������ģ������Ӹ���Ϊ1+8��![]() =2��Oԭ����4��λ�����ģ�2��λ�����ڣ��������Ӹ���Ϊ4��
=2��Oԭ����4��λ�����ģ�2��λ�����ڣ��������Ӹ���Ϊ4��![]() +2=4���ʾ�����ʵ�ʺ��е����������Ӹ�����Ϊ4��2�����Ը����ʻ�ѧʽΪVO2����þ�����ܶ�Ϊ
+2=4���ʾ�����ʵ�ʺ��е����������Ӹ�����Ϊ4��2�����Ը����ʻ�ѧʽΪVO2����þ�����ܶ�Ϊ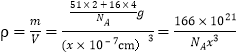 g/cm3��
g/cm3��

����Ŀ���״�����Ҫ�Ļ���ԭ�ϣ�����ú������������CO��CO2��H2����ȡ�״����л�������ķ�Ӧ�У�
��CO(g)+2H2(g)![]() CH3OH(g) ��H1=��99kJ��mol��1
CH3OH(g) ��H1=��99kJ��mol��1
��CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g) ��H2
CH3OH(g)+H2O(g) ��H2
������ʵĻ�ѧ�������������£�
��ѧ�� | C=O��CO2�� | H��H | C��O | H��O | C��H |
E/��kJ��mol��1�� | 803 | 436 | 343 | 465 | 413 |
��1���÷�Ӧ��H2=___________��
��2�����ڷ�Ӧ������˵������ȷ����___________��
A.�÷�Ӧ���κ��¶��¶����Է�����
B.�����¶ȣ�����Ӧ���������淴Ӧ���ʼ�С
C.ʹ�ô������������CO��ת����
D.����ѹǿ���÷�Ӧ�Ļ�ѧƽ�ⳣ������
��3����ij�¶��£���1.0moCO��2.0molH2����2L�Ŀո�ƿ�У�������Ӧ�����ڵ�5minʱ�ﵽ��ѧƽ��״̬����ʱ�״������ʵ�������Ϊ0.1���ڵ�10min��20minʱ�ֱ�ı䷴Ӧ�������״���Ũ���ڲ�ͬ�����µı仯״������ͼ��ʾ��

���ӷ�Ӧ��ʼ��5minʱ�����ɼ״���ƽ������Ϊ___________��
��H2��ƽ��ת������=___________%����ѧƽ�ⳣ��K=___________��
��1minʱ������___________����(������������С��������������)
��1mimʱ����___________4minʱ����(������������С��������������)
���Ƚϼ״���7��8min��12��13min��25��27minʱƽ����Ӧ����[ƽ����Ӧ���ʷֱ�����(7��8)����(12��13)����(25��27)��ʾ�Ĵ�С_________________________________��
��������ƿ����ͬ�ݻ��ľ����������ظ��������飬ƽ��ʱ�״������ʵ�������___________0.1��(����>������<������=��)



