��Ŀ����
������Ũ�ȶ�Ϊ0.1 mol/L��������Һ�У����루��ͨ�룩ij���ʺ�����Ӧ�Ⱥ�˳����ȷ����
| A���ں�Fe3����Cu2����H������Һ�м���п�ۣ�Cu2����Fe3����H�� |
| B���ں�I����SO32-��Br������Һ�в���ͨ��������I����Br����SO32- |
| C���ں�AlO2-��SO32-��OH������Һ����μ�������������Һ��OH����AlO2-��SO32- |
| D���ں�Fe3����H����NH4+����Һ�������ռ���Һ��H����NH4+��Fe3�� |
C
���������A��Zn������Fe3+��Ӧ������B������������SO32?��Ӧ������C����H+��Ӧ������OH��>AlO2->SO32-����ȷ��D���ں�Fe3����H����NH4+����Һ�������ռ���Һ��H����Fe3����NH4+������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
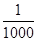 ����������CNO������
����������CNO������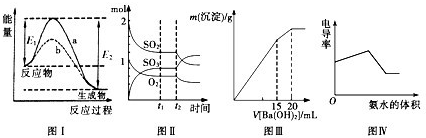
 2SO3���ɷֵ����ʵ����仯��t2ʱ�̸ı������������������SO2
2SO3���ɷֵ����ʵ����仯��t2ʱ�̸ı������������������SO2