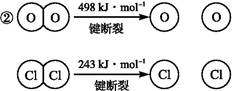
题目内容
围绕下列七种物质:①铝,②A12O3,③稀硝酸,④H2SO4,⑤Ba(OH)2固体,⑥氨水,⑦A12(SO4)3,根据要求回答下列问题。
(1)既能与强酸反应,又能与强碱反应的是 (填序号)。
(2)属于电解质的是 (填序号)。
(3)写出最适宜制取Al(OH)3的离子方程式 。
(4)两物质发生反应的离子方程式为H++OH—=H2O,请写出该反应的化学方程式 。
(5)34.2g⑦溶于水配成500mL溶液,溶液中SO42—的物质的量浓度为 。
(6)①与③发生反应的化学方程式为A1+4HNO3=A1(NO3)3+NO↑+2H2O,该反应中还原剂与氧化剂的物质的量之比是 。
(1)既能与强酸反应,又能与强碱反应的是 (填序号)。
(2)属于电解质的是 (填序号)。
(3)写出最适宜制取Al(OH)3的离子方程式 。
(4)两物质发生反应的离子方程式为H++OH—=H2O,请写出该反应的化学方程式 。
(5)34.2g⑦溶于水配成500mL溶液,溶液中SO42—的物质的量浓度为 。
(6)①与③发生反应的化学方程式为A1+4HNO3=A1(NO3)3+NO↑+2H2O,该反应中还原剂与氧化剂的物质的量之比是 。
(1)①②(共1分;答对1个给0.5分,答错一个不给分)
(2)②④⑤⑦(共2分;答对1个给0.5分,答错一个不给分)
(3)Al3++3NH3·H2O=Al(OH)3↓+3NH4+(共2分)
(4)2HNO3+Ba(OH)2= Ba(NO3)2+2H2O(共2分)
(5)0.6mol/L(共2分;单位未写扣0.5分)
(6)1∶1(共1分)
(2)②④⑤⑦(共2分;答对1个给0.5分,答错一个不给分)
(3)Al3++3NH3·H2O=Al(OH)3↓+3NH4+(共2分)
(4)2HNO3+Ba(OH)2= Ba(NO3)2+2H2O(共2分)
(5)0.6mol/L(共2分;单位未写扣0.5分)
(6)1∶1(共1分)
试题分析:(1)Al、Al2O3既能与强酸反应,又能与强碱反应;(2)纯净的酸、碱、盐、金属氧化物属于电解质,单质、酸溶液、碱溶液不是电解质;(3)实验室常用铝盐溶液与氨水制取Al(OH)3,Al3++3NH3·H2O=Al(OH)3↓+3NH4+;(4)H++OH—=H2O代表强酸与强碱结合生成可溶性盐和水的中和反应,2HNO3+Ba(OH)2= Ba(NO3)2+2H2O;(5)由m/M可知,n[A12(SO4)3]=0.1mol,由A12(SO4)3~3SO42—可知,n(SO42—)=0.3mol;由n/V可知,c(SO42—)=0.6mol/L;(6)铝元素全部由0价升高到+3价,被氧化,则Al是还原剂,1/4氮元素由+5价降低到+2价,被还原,则HNO3是氧化剂,则该反应中还原剂与氧化剂之比为1∶1。

练习册系列答案
 阅读快车系列答案
阅读快车系列答案
相关题目
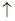
 Cl2
Cl2 2Cl2+2H2O,可实现氯的循环利用。
2Cl2+2H2O,可实现氯的循环利用。