��Ŀ����
����˵�����ʾ������ȷ���ǣ� ��
| A�����������������������ֱ���ȫȼ�գ����߷ų��������� |
B����C��ʯī�� C�����ʯ�� ��H="+1.9" KJ/mol��֪�����ʯ��ʯī�ȶ� C�����ʯ�� ��H="+1.9" KJ/mol��֪�����ʯ��ʯī�ȶ� |
| C��1mol H2������Cl2��ȼ�շ���183KJ����H2��ȼ����Ϊ183KJ�� |
| D����ϡ��Һ�У�H+(aq)+OH-(aq)==H2O(l)��H="-57.3" kJ/mol,������0.5 mol H2SO4��Ũ�����뺬1 mol NaOH����Һ��ϣ��������̷ų�����������57.3 kJ |
D
�������������A�����������������������ֱ���ȫȼ�գ�ǰ�߷ų��������࣬��Ϊ�����ת��Ϊ������Ҫ���ȡ�A����B�����ʯ����������ʯī�����ʯ����ʯī�ȶ���B����ȼ������1mol��ȼ����ȫȼ��ת��Ϊ�ȶ���������ʱ���ų���������C����D��ȷ����ΪŨ����ϡ�ͷ��ȡ�
���㣺��Ӧ�ȵķ������жϡ�

�����ʣ�ͨ��������ò������л��壩��һ�ֿ�������Դ���ܷ�������ͼת��������˵������ȷ����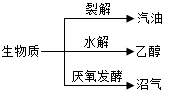
| A���������ڴ����� |
| B���Ҵ����ڿ�������Դ |
| C����������Ҫ�ɷ��Ǽ��� |
| D������������Դ��̫���� |
��֪��2CO(g)��O2(g)��2CO2(g) ��H����566 kJ/mol
Na2O2(s)��CO2(g)��Na2CO3(s)��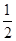 O2(g) ��H����226 kJ/mol
O2(g) ��H����226 kJ/mol
���������Ȼ�ѧ����ʽ�жϣ�����˵���������
| A��CO��ȼ����Ϊ283 kJ/mol |
B����ͼ�ɱ�ʾ��CO����CO2�ķ�Ӧ���̺�������ϵ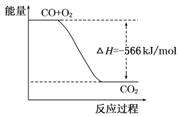 |
| C��2Na2O2(s)��2CO2(s)��2Na2CO3(s)��O2(g)����H����452 kJ/mol |
| D��CO(g)��Na2O2(s)��Ӧ�ų�509 kJ����ʱ������ת����Ϊ2��6.02��1023 |
��֪CH4(g)��2O2(g)=CO2(g)��2H2O(g)����H����Q1 ��
2H2(g)+O2(g)=2H2O(g) ��H=��Q2 ��
H2O(g)=H2O(l)�� ��H����Q3 ��
�����£�ȡ�����Ϊ4��1�ļ����H2�Ļ������112 L����״���£�������ȫȼ�պ�ָ������£���ų�������Ϊ��������
| A��4Q1��0.5Q2 | B��4Q1��Q2��10Q3 | C��4Q1��0.5Q2��9Q3 | D��4Q1��2Q2 |
���м���������ԭ��Ӧ���������ȷ�Ӧ���ǣ� ��
| A�������������������� |
| B��п����ϡH2SO4��Ӧ��ȡH2 |
| C��Ba(OH) 2·8H2O��NH4Cl��Ӧ |
| D��������̼����ȵ�̿��Ӧ����һ����̼ |
��֪����N2(g)��O2(g)=2NO(g)����H1����180 kJ·mol��1
��N2(g)��3H2(g)  2NH3(g)����H2����92.4 kJ·mol��1
2NH3(g)����H2����92.4 kJ·mol��1
��2H2(g)��O2(g)=2H2O(g)����H3����483.6 kJ·mol��1
����˵����ȷ����( ��)
A����Ӧ���е������仯��ͼ��ʾ����H2��E1��E3 |
| B��H2��ȼ����Ϊ241.8 kJ·mol��1 |
| C���ɷ�Ӧ��֪���¶�һ���������£���һ�����ܱ�������ͨ��1 mol N2��3 mol H2����Ӧ��ų�������ΪQ1 kJ����ͨ��2 mol N2��6 mol H2��ַ�Ӧ��ų�������ΪQ2 kJ����184.8>Q2>2Q1 |
| D�����Ĵ�������ӦΪ4NH3(g)��5O2(g)=4NO(g)��6H2O(g)��H����906 kJ·mol��1 |
��֪�к��ȵ���ֵ��57.3KJ/mol�����з�Ӧ����ʱ����������������57.3KJ ����
| A��500mL1 mol/LHCl��aq����500mL 1mol/LNaOH��aq�� |
| B��500mL 1mol/LH2SO4��aq����500mL 1mol/LBa��OH��2��aq�� |
| C��1000mL1.0mol/L��CH3COOH��aq����1000mL1.0mol/L��NaOH��aq�� |
| D��1000mL1.0mol/L��HCl��aq����1000mL1.0mol/L��NaOH��aq�� |
�����Ȼ�ѧ����ʽ��д��ȷ���ǣ���H�ľ���ֵ��Ϊ��ȷֵ��
| A��2NO2��O2��2NO��H����116.2 kJ/mol����Ӧ�ȣ� |
| B��S(s)��O2(g)��SO2(g)��H����296.8 kJ/mol����Ӧ�ȣ� |
| C��NaOH(aq)��HCl(aq)��NaCl(aq)��H2O(l)��H����57.3 kJ/mol���к��ȣ� |
| D��C2H5OH(l)��2O2(g)��2CO (g)��3H2O(g)��H����801 kJ/mol��ȼ���ȣ� |